| Identification | Back Directory | [Name]
(Pentamethylcyclopentadienyl)iridium(III) chloride dimer | [CAS]
12354-84-6 | [Synonyms]
iridium(III)
Dichloropentamethylcyclopentadienyliridiumdimer
pentamethylcyclopentadienyliridium(iii) chloride
Dichloro(pentamethylcyclopentadienyl)iridium(III)
Pentamethylcyclopentadienyliridium(Ⅲ) chloride dimer
Dichloro(pentaMethylcyclopentadienyl)iridiuM(III)diMMer
DICHLORO(PENTAMETHYLCYCLOPENTADIENYL)IRIDIUM(III) DIMER
(PENTAMETHYLCYCLOPENTADIENYL)IRIDIUM(III) CHLORIDE DIMER
Dichloro(pentamethylcyclopentadienyl)iridium(III)dimer,98%
(Pentamethylcyclopentadienyl)iridium(III) Dichloride Dimer
PentaMethylcyclopentadienyliridiuM(III) chloride,diMer 96%
Pentamethylcyclopentadienyl)iridium(III) chloride dimer,98%
(Pentamethylcyclopentadienyl)iridium(III) chloride dimer,99%
(PentaMethylcyclopentadienyl)iridiuM(III) chloride diMer, 99% 250MG
PentaMethylcyclopentadienyl)iridiuM(III) chloride diMer,(Cp*IrCl2)2
PentaMethylcyclopentadienyl)iridiuM(III) chloride diMer,98% (Cp*IrCl2)2
PentaMethylcyclopentadienyliridiuM(Ⅲ) chloride diMer, 48% Ir, Product of UMicore | [Molecular Formula]
C20H30Cl4Ir2 10* | [MDL Number]
MFCD00075435 | [MOL File]
12354-84-6.mol | [Molecular Weight]
796.7 |
| Chemical Properties | Back Directory | [Appearance]
orange fine crystalline powder | [Melting point ]
245 °C (decomp)(Solv: chloroform (67-66-3); hexane (110-54-3)) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
crystal | [color ]
orange | [Stability:]
Moisture Sensitive | [CAS DataBase Reference]
12354-84-6 |
| Hazard Information | Back Directory | [Chemical Properties]
orange fine crystalline powder | [Uses]
Dichloro(pentamethylcyclopentadienyl)iridium(III) dimer is used as a precursor to catalysts for the asymmetric transfer hydrogenation of ketones. It is a catalyst for greener amine synthesis. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. | [reaction suitability]
core: iridium
reagent type: catalyst |
| Questions And Answer | Back Directory | [Reaction]
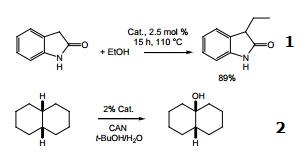
- Iridium-catalyzed C-3 alkylation of oxindole with alcohols.
- Precursor to N-heterocyclic carbene catalyst effective for hydrogenation and alkylation of amines and alcohols.
- Precursor to efficient phosphine free catalyst for enantioselective hydrogenation of quinoline derivatives.
- Catalyst for oxidative C–H activation.
- Precursor to an effective water oxidation catalyst.
|
|
|