| Identification | Back Directory | [Name]
Omarigliptin (MK-3102) | [CAS]
1226781-44-7 | [Synonyms]
MK-3102
MK-3012
OMarigliptin
MK-3102, >=98%
MK-3102 - M10588
OMarigliptin (MK-3102)
(2R,3S,5R)-2-(2,5-Difluorophenyl)-5-(2-(methylsulfonyl)pyrrolo[3,4-c]pyrazol-5(2H,4H,6H)-yl)te
(2R,3S,5R)-2-(2,5-difluorophenyl)-5-(2-(Methylsulfonyl)pyrrolo[3,4-c]pyrazol-5(2H,4H,6H)-yl)tetrahydro-2H-pyran-3-aMine
(2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine
MK-3102
(2R,3S,5R)-2-(2,5-difluorophenyl)-5-(2-(Methylsulfonyl)pyrrolo[3,4-c]pyrazol-5(2H,4H,6H)-yl)tetrahydro-2H-pyran-3-aMine
(2S,3R,5S)-2-(2,5-Difluorophenyl)-5-[2-(Methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-aMine (MK-3102 enantioMer)
2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine MK3102 | [EINECS(EC#)]
682-558-0 | [Molecular Formula]
C17H20F2N4O3S | [MDL Number]
MFCD22573261 | [MOL File]
1226781-44-7.mol | [Molecular Weight]
398.43 |
| Chemical Properties | Back Directory | [Boiling point ]
529.4±60.0 °C(Predicted) | [density ]
1.61±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C(protect from light) | [solubility ]
insoluble in EtOH; insoluble in H2O; ≥17.15 mg/mL in DMSO | [form ]
solid | [pka]
9.11±0.60(Predicted) | [color ]
White to off-white | [InChI]
InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 | [InChIKey]
MKMPWKUAHLTIBJ-ISTRZQFTSA-N | [SMILES]
[C@H]1(C2=CC(F)=CC=C2F)OC[C@H](N2CC3=CN(S(C)(=O)=O)N=C3C2)C[C@@H]1N | [CAS DataBase Reference]
1226781-44-7 |
| Hazard Information | Back Directory | [Description]
Merck earned its first global
approval for omarigliptin in Japan in 2015, and phase III
development is ongoing in other countries around the globe for
this interesting small molecule DPP-4 inhibitor. Interestingly,
while most DPP-4 inhibitors used to treat type 2 DM require
daily administration, omarigliptin is a weekly treatment. The
process-scale synthesis of omarigliptin has been nicely
described in an October 2015 paper from the Merck process
group. | [Description]
MK-3102 is a potent, reversible, and competitive inhibitor of dipeptidyl peptidase 4 (DPP-4; IC50 = 1.6 nM; Ki = 0.8 nM).1 It is selective for DPP-4 over 168 proteases, ion channels, and enzymes with IC50 values greater than 10 μM in all assays. MK-3102 significantly reduces blood glucose levels in a dose-dependent manner in vivo in rats. It also has a long half-life (11 and 22 hours in rat and dog, respectively) making it suitable for once weekly dosing. Clinical trials demonstrate that formulations containing MK-3102 reduce plasma glucose and HbA1c in patients with type 2 diabetes mellitus (T2DM). | [Uses]
Omarigliptin is a potent and selective dipeptidyl peptidase 4 (DPP-4) inhibitor to be used as treatment for type 2 diabetes. | [Definition]
ChEBI: Omarigliptin is a pyrrolopyrazole. | [Synthesis]
The synthesis began with the efficient condensation of
pyrrolidinone 149 with dimethylformamide-dimethylacetal
(DMF-DMA) to afford enaminoketone 150 in 88% yield. Subsequent condensation with hydrazine
monohydrate gave tertiary alcohol 151 in 92% yield, and this
step was followed by acid-promoted dehydration to afford fused
pyrazole 152. An initial kinetic mesylation delivered a 1:5 ratio
of 147:153, in favor of the undesired regioisomer. However,
when the crude mixture was warmed to ambient temperature
and treated with potassium tert-butoxide, thermodynamic
equilibration provided the more stable N1-mesylate 147. This
process furnished the desired regioisomer 147 in a 30:1 ratio
and 84% yield over the two steps. Reaction
monitoring by HPLC suggests that cleavage of the mesyl group
of 153 results in anion formation on the adjacent nitrogen,
which then allows for mesylation at the desired position.

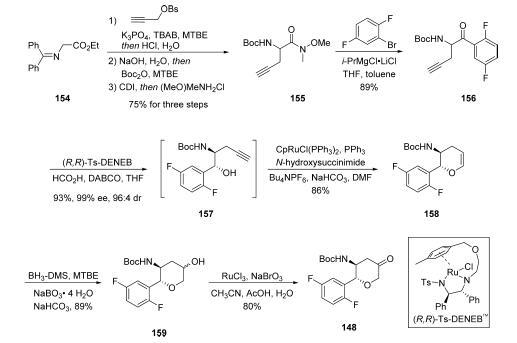
Ester 154 was subjected to a
three-step sequence whereby alkylation with propargyl besylate
followed by saponification with sodium hydroxide and Boc
protection resulted in amide 155 in 75% yield over three steps.
The Weinreb amide was then subjected to the Knochel “turbo
Grignard” reagent derived from 1-bromo-2,4-difluorobenzene
to provide ketone 156 in 89% yield. An enantioselective
transfer hydrogenation was carried out utilizing (R,R)-Ts-
DENEB as the chiral induction reagent to afford intermediate
157 in excellent yield and enantio- and diastereoselectivity,
which underwent ruthenium-mediated cyclization with the
pendant alkyne to afford dihydropyran 158 in 86% yield. A
two-step hydroboration/oxidation involving the endocyclic
vinyl ether furnished 159 as a mixture of diastereomers in
89% yield, and this was followed by RuCl3/NaBO3-mediated
oxidation to provide the lactone fragment 148 in 80% yield.
Removal of the Boc group within 147 was
effected upon treatment with TFA, affording intermediate 160,
which was not isolated but instead exposed to ketone 148
under reductive amination conditions to afford diaminopyran 161 in excellent yield and diastereoselectivity (30:1 dr). Finally,
Boc deprotection and crystallization from THF/heptanes
furnished omarigliptin in an impressive 45% yield over its
nine-step longest linear sequence.
 | [in vitro]
mk-3102 is a competitive, reversible inhibitor of dpp-4 and is more potent than sitagliptin. it is highly selective over all proteases tested, including qpp, fap, pep, dpp8, and dpp9. the compound has weak ion channel activity [1]. | [in vivo]
mk-3102 was evaluated for its ability to improve glucose tolerance in lean mice. when orally administered 1 h prior to dextrose challenge in an oral glucose tolerance test, it significantly reduced blood glucose excursion in a dosedependent manner from 0.01 mg/kg to 0.3 mg/kg [1]. | [storage]
Store at -20°C | [References]
[1] biftu t, sinha-roy r, chen p, qian x, feng d, kuethe jt, scapin g, gao yd, yan y, krueger d, bak a, eiermann g, he j, cox j, hicks j, lyons k, he h, salituro g, tong s, patel s, doss g, petrov a, wu j, xu ss, sewall c, zhang x, zhang b, thornberry na, weber ae. omarigliptin (mk-3102): a novel long-acting dpp-4 inhibitor for once-weekly treatment of type 2 diabetes. j med chem. 2014 apr 24;57(8):3205-12. |
| Questions And Answer | Back Directory | [Oral hypoglycemic agents]
Omarigliptin (MK-3102) is an oral hypoglycemic agent of super long-lasting dipeptidyl peptidase-4 (DPP-4) developed by Merck & Co., USA. It is administered orally once a week, being able to produce sustained DPP-4 inhibition with a new mechanism of lowering blood sugar. Meanwhile, it does not increase body weight and not cause hypoglycemia and edema. Its mechanism of action is through inhibiting the degrading of the in vivo dipeptidyl peptidase-4 (DPP-4) on the GLP-1, prolonging the action time of glucagon-like peptide-1 (GLP-1), thereby increasing the blood concentration of the endogenous GLP-1 and GIP, and ultimately improving blood glucose control.
GLP-1 is a class of incretin, belonging to a moderate-length straight-chain peptide hormone with insulin secretion promoting effect generated in the intestine. Its action features include: merely postprandial production, promoting insulin secretion by islet β-cells and further lowing the blood sugar level but without triggering the hypoglycemia; it can inhibit the pancreatic α cells from secreting the glucagon; delay the gastric emptying which is good for the postprandial blood glucose control; it can cause inhibition of intestinal secretion of lipoproteins and may reduce the postprandial hyperglycemia which is risk factors for cardiovascular disease, thus playing a heart protection effect.
Omarigliptin is a kind of DPP-4 inhibitor oral hypoglycemic agents administrated once weekly, improving the drug compliance. Poor adherence to medication is a common problem in clinical practice, particularly in chronic asymptomatic diseases such as type 2 diabetes, dyslipidemia, and hypertension. For the patients of type II diabetes, improving the adherence to medication is critical to maintaining good glycemic control during long-term treatment, thereby preventing the development of type 2 diabetes and the development and progression of complications.
On September 19, 2014, Merck published the first data on the Phase III clinical development of omarigliptin in the treatment of type 2 diabetes at the 50th annual European Diabetes Association (EASD) annual meeting. Compared with placebo, omarigliptin can significantly reduce the HbA1c levels, meanwhile being able to achieve equal efficacy and tolerability to a 50-mg daily dose of the DPP-4 inhibitor Januvir (Sigma).
In December 2014, Merck has submitted an experimental new drug application (NDA) of the hypoglycemic drug omarigliptin to the Japanese Pharmaceutical and Medical Device Agency (PMDA), marking the world's first regulatory application. | [Clinical evaluation]
(1) Pharmacodynamic properties. The inhibitory activity of Omarigliptin on DPP-4 (C50 = 1.6 nM, K1 = 0.8 nM) is stronger than that of sitagliptin (IC50 = 18 nM).
(2) High selectivity to DPP-4. DPP has many subtypes from 1-9, of which the inhibition constant of DPP-8 and DPP-9 are often related with the toxic effects of drugs. Two weeks of rat studies have shown that inhibition of DPP-8 and DPP-9 can lead to increased hair loss, thrombocytopenia, anemia, splenomegaly and even death cases in rats. In acute dog toxicity studies, inhibition resulted in bloody diarrhea in dogs. In vitro studies have shown that Omarigliptin is very specific for DPP-4 inhibition and has very low activity against the remaining proteases (including QPP, FAP, FEF, DPP8 and DPP9) with IC50> 67 μM and thus does not produce the above-mentioned side effects.
(3) In the aspect of pharmacokinetics, the most significant feature of Omarigliptin is a long half-life of up to 63h while the value for sitagliptin is 12.4h, for vildagliptin is 2-3 h and for saxagliptin in the parent body is 2.5 h. This means that Omarigliptin only needs to be taken once a week to achieve better glycemic control effect.
(4) In the aspect of usage range, renal insufficiency has effects on the in vivo exposure level and clearance rate of the omarigliptin, so we may need to adjust the dosage and usage of the drug. This may be similar to other DPP-4 inhibitors such as sitagliptin, vildagliptin and saxagliptin.
Comparatively, the advantage of omarigliptin is obvious, which has excellent PK/PD, efficacy and safety with excellent potential during the clinical treatment. | [Market expectation]
Diabetes is caused by the effects of genetic factors, immune dysfunction, microbial infection and toxins, free radical toxins, mental factors, and various kinds of pathogenic factors on the body that lead to islet dysfunction, insulin resistance, further causing a series metabolic disorders of sugar, protein, fat, water and electrolytes. It is clinically mainly characterized by the high blood sugar. In typical cases, there may be polyuria, polydipsia, more food, weight loss and other performance, namely "three polys and one little" symptoms, Blood sugar, once not controlled well, will lead to poor diabetes complications, leading to the failure of lesions in kidney, eye, foot and other parts and can’t be cured.
China is a big country of diabetes. According to the latest research results, in the samples of China's 18-year-old and adult, the estimated prevalence of diabetes was 11.6%, about 113.9 million people. The pre-diabetes (IGT) prevalence rate in Chinese adults is about 50.1%, namely, half of the people is the reserve of diabetes. This figure is very alarming, indicates that China's diabetes situation is very serious.
DPP-4 inhibitors are the host spots at both home and abroad in recent years. The number listed abroad has already reached 7. There are also a number of domestic 1.1 class novel drug of independent research and development having obtained clinical approval documents. The first marketed DPP-4 inhibitor is sitagliptin, with sales currently being $ 4 billion and is expected to reach $ 7.8 billion by 2018.
The pharmacological effects, clinical evaluation, indications, and market prospects of oral hypoglycemic agents, omarigliptin, are compiled by the editor, tontong of the Chemicalbook (2015-09-22). |
|
|