| Identification | Back Directory | [Name]
Trifluoperazine | [CAS]
117-89-5 | [Synonyms]
rp7623
RP 7623
modalina
Triflurin
Triftazin
Triftazine
Triphtazin
Stellazine
Terfluzine
Triphtazine
Triperazine
Jatroneural
Triphthasine
Triphthazine
Fluoperazine
Trifluoperazin
Trifluperazine
TRIFLUOPERAZINE
Trifluoperazina
Trifluroperizine
Trifluoroperazine
Trifluoromethylperazine
Trifluoperazine (base and/or unspecified salts)
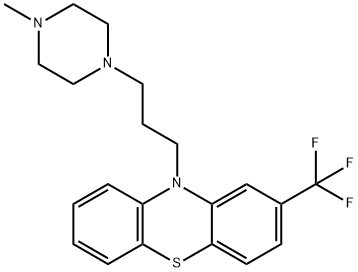
Trifluoromethyl-10-(3'-(1-methyl-4-piperazinyl)propyl)phenothiazine
trifluoromethyl-10-(3’-(1-methyl-4-piperazinyl)propyl)phenothiazine
2-trifluoromethyl-10-[3’-(1-methyl-4-piperazinyl)propyl]phenothiazine
10-(gamma-(n’-methylpiperazino)propyl)-2-trifluoromethylphenothiazine
10-(gamma-(n’-methylpiperazino)propyl)-2-trifluoromethylphenothiozine
10-(gamma-(N'-Methylpiperazino)propyl)-2-trifluoromethylphenothiozine
10-[3-(4-methylpiperazin-1-yl)-propyl]-2-trifluoromethylphenothiazine
10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)-phenothiazin
10-(3-(4-Methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)phenothiazine
Phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-
10-[3-(4-Methylpiperazin-1-yl)propyl]-2-trifluoromethyl-10H-phenothiazine
10-[3-(4-Methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine
10H-Phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)- | [EINECS(EC#)]
204-219-4 | [Molecular Formula]
C21H24F3N3S | [MDL Number]
MFCD00242601 | [MOL File]
117-89-5.mol | [Molecular Weight]
407.5 |
| Chemical Properties | Back Directory | [Melting point ]
232°C | [Boiling point ]
bp0.6 202-210° | [density ]
1.2060 (estimate) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly), Water (Slightly) | [form ]
Solid | [pka]
pKa 8.05±0.04(H2O
t = 20 0.5
I not reported
but pKa
was stated
to be
independent
of I.) (Uncertain) | [color ]
White to Off-White | [Water Solubility ]
12.23mg/L(24 ºC) | [NIST Chemistry Reference]
Trifluoperazine(117-89-5) |
| Hazard Information | Back Directory | [Originator]
Stelazine,SKF,US,1958 | [Uses]
10-[3-(4-Methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine is a psychoactive drug. | [Uses]
Antipsychotic. | [Uses]
Trifluoperazine is one of the most active antipsychotic drugs. A moderate stimulatory
effect accompanies the neuroleptic effect. Trifluoperazine is unique in that, patients instead
of the usual stiffness and weakness characteristic of phenothazine derivatives, become
more lively. This drug has a strong anticonvulsant activity. It is widely used in psychiatry
for treating schizophrenia and other mental illnesses. | [Definition]
ChEBI: Trifluoperazine is a member of the class of phenothiazines that is phenothiazine having a trifluoromethyl subsitituent at the 2-position and a 3-(4-methylpiperazin-1-yl)propyl group at the N-10 position. It has a role as a dopaminergic antagonist, an antiemetic, an EC 1.8.1.12 (trypanothione-disulfide reductase) inhibitor, an EC 5.3.3.5 (cholestenol Delta-isomerase) inhibitor, a calmodulin antagonist and a phenothiazine antipsychotic drug. It is a N-alkylpiperazine, a N-methylpiperazine, a member of phenothiazines and an organofluorine compound. | [Manufacturing Process]
A mixture of 17.2 grams of 2-trifluoromethylphenothiazine, 3.1 grams of
sodamide and 14 grams of 1-(3'-chloropropyl)-4-methylpiperazine in 200 ml
of xylene is heated at reflux for 2 hours. The salts are extracted into 150 ml
of water. The xylene layer is then extracted with several portions of dilute
hydrochloric acid. The acid extracts are combined and neutralized with
ammonium hydroxide solution. The product, 10-[3'-(4''-methyl-1''-
piperazinyl)-propyl]-2-trifluoromethylphenothiazine, is taken into benzene and
purified by vacuum distillation, BP 202° to 210°C at 0.6 mm. | [Therapeutic Function]
Tranquilizer | [Clinical Use]
Schizophrenia and other psychoses
Anxiety
Severe nausea and vomiting | [Synthesis]
Trifluoperazine, 2-trifluoromethyl-10-[3-(4-methyl-1-piperazinyl) propyl]-
phenothazine (6.1.5), is synthesized in the manner described above of alkylation using 2-
trifluoromethylphenothazin-4-methyl-1-piperazinylpropylchloride [11,17–20]. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval, e.g. procainamide, disopyramide,
dronedarone and amiodarone - avoid with
amiodarone and dronedarone.
Antibacterials: increased risk of ventricular
arrhythmias with delamanid and moxifloxacin -
avoid with moxifloxacin.
Antidepressants: increased level of tricyclics; possibly
increased risk of antimuscarinic side effects; risk
of ventricular arrhythmias with citalopram and
escitalopram - avoid; possible increased risk of
convulsions with vortioxetine.
Antiepileptics: antagonism (convulsive threshold
lowered).
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol and pimozide - avoid;
possible increased risk of ventricular arrhythmias
with risperidone.
Antivirals: concentration possibly increased with
ritonavir; increased risk of ventricular arrhythmias
with saquinavir - avoid.
Anxiolytics and hypnotics: increased sedative effects.
Atomoxetine: increased risk of ventricular
arrhythmias.
Beta-blockers: enhanced hypotensive effect;
increased risk of ventricular arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Diuretics: enhanced hypotensive effect.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity.
Pentamidine: increased risk of ventricular arrhythmias. | [Metabolism]
Trifluoperazine undergoes extensive first pass metabolism. The major metabolite is the possibly active N-oxide; other metabolites include the sulfoxide and the 7-hydroxy derivative.
Elimination occurs in the bile and urine. |
|
|