| Identification | Back Directory | [Name]
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione | [CAS]
1150560-59-0 | [Synonyms]
EOS-61868
Eelagolix-1
Eelagolix int1
Elagolix Impurity 6
Elagolix Impurity 17
Eelagolix intermediate
elagolix intermediate 6
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl...
5-(2-fluoro-3-methoxy-phenyl)-1-(2-fluoro-6-trifluoromethyl-benzyl)-6-methyl-1H-pyrimidine-2,4- dione
5-(2-Fluoro-3-methoxyphenyl)-1-[2-fluoro-6-(trifluoromethyl)benzyl]-6-methyl-2,4(1H,3H)-pyrimidinedione
5-(2-fluoro-3-methoxyphenyl)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methy-6-methyl-2,4(1H,3H)-pyrimidinedione
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione
2,4(1H,3H)-Pyrimidinedione, 5-(2-fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-
5-(2- Fluoro -3- methoxyphenyI )-1-[[2- fluoro -6-( trifluoromethyl ) phenyl ] methyl ]-6- methyl -2,4(1H,3H)- pyrimidinedione
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione ISO 9001:2015 REACH | [Molecular Formula]
C20H15F5N2O3 | [MDL Number]
MFCD30570283 | [MOL File]
1150560-59-0.mol | [Molecular Weight]
426.337 |
| Hazard Information | Back Directory | [Uses]
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione can be used as an intermediate in organic synthesis Body and pharmaceutical intermediates are mainly used in laboratory research and development processes and chemical production processes. | [Uses]
5-?(2-?Fluoro-?3-?methoxyphenyl)?-?1-?[[2-?fluoro-?6-?(trifluoromethyl)?phenyl]?methyl]?-?6-?methyl-?2,?4(1H,?3H)?-?pyrimidinedione is an intermediate in the synthesis of Elagolix, a gonadotropin-releasing hormone antagonist (GnRH) used in the treatment of endometriosis. | [Synthesis]
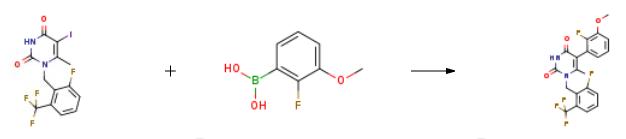
To a reactor was charged l-(2-fluoro-6-trifluoromethyl-benzyl)-5-iodo-6-methyl- lH-pyrimidine-2,4-dione Ib (5.0 kg), 2-fluoro-3-methoxyphenylboronic acid (2.58 kg), and acetone (5.5 L). The mixture was agitated and a potassium hydroxide/water solution (2.658 kg/19.0 L) was charged. The reactor contents were degassed for 30-60 min, then the internal temperature was adjusted to 40 0C. l,l-(bis-di-t-butylphosphino)ferrocene palladium dichloride (11.4 g) was added to the reactor and the contents mixed with jacket temperature set to 45 0C until the reaction was complete (2.5 hr). The reaction mixture was cooled to 20-30 0C. Celite (1.25 kg) was charged to the reactor and stirred for more than one hour and the mixture was filtered through a Celite pad (0.51 kg). The reactor and Celite cake were washed with acetone/water/KOH (2.6 L/7.5 L/0.38 kg). The filtered solutions were passed through a line filter and added over a period of 1-1.5 hr to a mixture of THF/AcOH/Water (15.0 L/7.53 L/5.0 L) maintained at 62 0C. The reactor contents were cooled to 20 0C over 2-3 hr. The mixture was filtered and the cake washed with 60:40 water/MeOH (2 x 12.6 L) followed by methanol (2 x 16 L). The solid was dried in a vacuum oven at 65 0C for 18 hr to provide 5-(2-fluoro-3-methoxy- phenyl)-l-(2-fluoro-6-trifluoromethyl-benzyl)-6-methyl-lH-pyrimidine-2,4-dione Ic (4.312 kg, 87 % molar yield) as an off-white solid. LCMS (ESI) m/z 427.1 (MH+). If necessary, the material may be solubilized, re-treated with Celite, and crystallized as above to increase purity. |
|
|