| Identification | More | [Name]
1-Pyrrolidino-1-cyclohexene | [CAS]
1125-99-1 | [Synonyms]
1-(1-CYCLOHEXEN-1-YL)PYRROLIDINE
1-(1-PYRROLIDINO)CYCLOHEXENE
1-(CYCLOHEXEN-1-YL)PYRROLIDINE
1-PYRROLIDINO-1-CYCLOHEXENE
1-PYRROLIDINO-2-CYCLOHEXENE
N-(1-CYCLOHEXEN-1-YL)-PYRROLIDIN
N-(1-CYCLOHEXEN-1-YL)PYRROLIDINE
1-(1-Cyclohexenyl)pyrrolidine
1-(1-Pyrrolidino)-1-cyclohexene
1-(1-Pyrrolidinyl)cyclohexene
1-Pyrrolidinocyclohexene
1-Pyrrolidinyl-1-cyclohexene
Cyclohexanone pyrrolidine enamine
N-(1-Cyclohexenyl)pyrrolidine
N-Pyrrolidino-1-cyclohexene
N-(cyclohex-1-en-1-yl)pyrrolidine
1-(1-PYRROLIDINO)CYCLOHEXENE 97%
n-(cyclohexen-1-yl)pyrrolidine
1-Cyclohex-1-en-1-ylpyrrolidine
1-cyclohexenylpyrrolidine | [EINECS(EC#)]
214-414-6 | [Molecular Formula]
C10H17N | [MDL Number]
MFCD00003163 | [Molecular Weight]
151.25 | [MOL File]
1125-99-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 1993 3/PG 3
| [WGK Germany ]
3
| [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29339980 |
| Hazard Information | Back Directory | [Chemical Properties]
clear light yellow liquid | [Uses]
1-Pyrrolidino-1-cyclohexene is a cyclic enamine that is used as an electron-rich dienophile in synthetic reactions. | [Synthesis Reference(s)]
Chemical and Pharmaceutical Bulletin, 16, p. 1466, 1968 DOI: 10.1248/cpb.16.1466 | [Reactivity Profile]
The enamine nitrogen and its β-carbon atom are nucleophilic, undergoing substitution or conjugate addition; they can take part in 1,2- or 1,4-cycloadditions; iminium salts act as electrophiles.
| [Synthesis]
The most commonly used preparation method for these enamines is the acid-catalyzed condensation of a secondary amine with cyclohexanone. An example is the reaction of Pyrrolidine with cyclohexanone (eq 1). Using a preformed Titanium(IV) Chloride-amine complex with cyclohexanone greatly decreases the reaction time while maintaining high yields. This technique is also effective with functionalized cyclohexanones. When unsymmetrical tin(II) amides are allowed to react with cyclohexanone, good yields of enamines result.
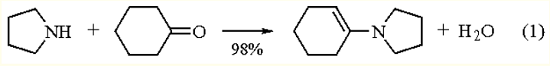
| [Precautions]
Readily reacts with water or atmospheric oxygen; should be stored under dry nitrogen.
|
|
|