| Identification | More | [Name]
1,4-Diazacyclohexane | [CAS]
110-85-0 | [Synonyms]
1,4-DIAZACYCLOHEXANE
AKOS 90646
AKOS BBS-00004315
DIETHYLENEDIAMINE
HEXAHYDRO-1,4-DIAZINE
HEXAHYDROPYRAZINE
PIPERAZINE
1,4-Diethylenediamine
1,4-Piperazine
Antepan
Antiren
Asca-Trol No. 3
Diethyleneimine
Dispermine
Eraverm
Hexahydropyrazin
hexahydro-pyrazin
Lumbrical
Piperazidine
Piperazin | [EINECS(EC#)]
203-808-3 | [Molecular Formula]
C4H10N2 | [MDL Number]
MFCD00005953 | [Molecular Weight]
86.14 | [MOL File]
110-85-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Solid | [Melting point ]
109-112 °C(lit.)
| [Boiling point ]
146 °C | [bulk density]
400kg/m3 | [density ]
1,1 g/cm3 | [vapor pressure ]
0.8 mm Hg ( 20 °C)
| [FEMA ]
4250 | PIPERAZINE | [refractive index ]
1.4460 | [Fp ]
65 °C
| [storage temp. ]
Store in dark! | [solubility ]
H2O: 0.1 M at 20 °C, clear, colorless
| [form ]
Crystalline Flakes | [pka]
9.83(at 23℃) | [color ]
White to slightly yellow | [Odor]
at 0.10 % in dipropylene glycol. ammoniacal | [PH]
11.0-12.5 (25℃, 0.1M in H2O) | [Stability:]
Stable. Hygroscopic. Light sensitive. Flammable. Incompatible with strong oxidizing agents. | [biological source]
rabbit | [explosive limit]
14% | [Odor Type]
ammoniacal | [Water Solubility ]
150 g/L (20 ºC) | [Sensitive ]
Air Sensitive & Hygroscopic | [λmax]
λ: 260 nm Amax: 0.035
λ: 280 nm Amax: 0.010 | [JECFA Number]
1615 | [Merck ]
14,7464 | [BRN ]
102555 | [Exposure limits]
ACGIH: TWA 0.03 ppm | [Contact allergens]
Piperazine is contained in pyrazinobutazone, an equimolar
salt of piperazine and phenylbutazone. Among occupational
cases, most were reported in the pharmaceutical
industry or laboratory workers, in nurses, and in
veterinarians. | [InChIKey]
GLUUGHFHXGJENI-UHFFFAOYSA-N | [LogP]
-1.24 at 20-25℃ | [CAS DataBase Reference]
110-85-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Piperazine(110-85-0) | [EPA Substance Registry System]
110-85-0(EPA Substance) |
| Hazard Information | Back Directory | [Definition]
ChEBI: An azacycloalkane that consists of a six-membered ring containing two nitrogen atoms at opposite positions. | [General Description]
Needle-like white or colorless crystals. Shipped as a solid or suspended in a liquid medium. Very corrosive to skin, eyes and mucous membranes. Solid turns dark when exposed to light. Flash point 190°F. Used as a corrosion inhibitor and as an insecticide. | [Reactivity Profile]
PIPERAZINE(110-85-0) neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Absorbs carbon dioxide from the air, which can cause dry crystals to seem to melt. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. This compound is sensitive to light; PIPERAZINE(110-85-0) absorbs water and carbon dioxide from air. This compound may be corrosive to aluminum, magnesium and zinc. . | [Air & Water Reactions]
Flammable. Absorbs water and carbon dioxide from air. Soluble in water. | [Health Hazard]
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | [Potential Exposure]
(Piperazine): Primary irritant (w/o allergic reaction), | [Fire Hazard]
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water, or milk. Do not induce vomiting. Medical observation is recommended for 24�48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN2579 Piperazine, Hazard class: 8; Labels: 8-Corrosive material. | [Incompatibilities]
Aqueous solution is a strong base. Violent reaction with strong oxidizers and dicyanofurazan. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrogen compounds, carbon tetrachloride. Attacks aluminum, copper, nickel, magnesium and zinc. | [Description]
Piperazine is contained in pyrazinobutazone, an equimolecular
sah of piperazine and phenylbutazone.
Among occupational cases, most were reported in the
pharmaceutical industry or laboratory, in nurses and
in veterinarians. | [Chemical Properties]
Colorless to yellow solid; salty taste. | [Chemical Properties]
Piperazine is white to cream-colored needles or powder. Characteristic ammonia-like odor. Combustible solids that do not easily ignite. | [Uses]
keratolytic, antiseborheic | [Uses]
Labelled Piperazine | [Uses]
Piperazine is used as an intermediate in themanufacture of dyes, pharmaceuticals, polymers,surfactants, and rubber accelerators. | [Brand name]
Pincets (Marion Merrell Dow);
Pinsirup (Marion Merrell Dow);Adelmintex;Adipalis;Adipalit;Adiver;Ancaris thenium;Ancazine;Antelmina;Antepar (b-w);Anterobius;Anthalazine;Anthelmina;Anticucs;Antivermine;Ascalix;Ascarinex;Ascarivet;Asca-trol no.3;Asepar;Askaripar;Averamexan;Bel-zine;Bioxurin;B-piperazine;Brirel;Candizine;Carudol;Ciperazin;Citrazine;Coopane;Dak;Demovermil;Diatesurico;Dicevermin;Digesan;Dilaurazine;Dispermin;Diurazina;Dowzene;Ecosan;Endorid;Entazin;Equizole-a;Escovermin;Esteropipate;Etaphylline (acetyllinate);Gentiazina;Glycopiparsol;Heksapar;Helmacid;Helmezin;Helmicide;Helmifren;Helmipar;Helmirazine (adipate);Helmirazine (citrate);Helmitin;Helmizin;Herb royal round worm treatment;Hexanthelin;Ismiverm;Janes liquid permifu;Jarabe neox;Jetsan supp. (adipate);Justalmin;Kennel-maid;Kihomato;Kontipar;Lamboxil;Lombricida tropico;Lombrifher;Lombrikal;Lombrimade;Mapiprin;Maskito;Noxiurotan;Ogen;Okuside;Optiverm;Oxiril syrup (hydrate);Oxiuran (hydrate);Oxiurasin;Oxiustip elix;Oxivermin;Oxizin;Oxucid;Oxuril;Oxypip;Oxyzin;P.c. (citrate);Padrax;Paravermin;Pariamate;Par-tega;Perin;Piavermit;Pincide;Pipan;Pip-a-ray;Pipenin;Piperacid;Piperamicin;Piperascat;Piperaskat;Piperate;Piperaverm;Piperazinal;Piperazine (adipate);Pipercrean;Piperex;Piperiod;Piperital od;Piperitol;Piper-jodina;Piperol fort;Piperone;Piperoverm;Pipertox;Piperver;Piperzinal;Pipeverm;Pipezol;Pipizan citrate;Pipracid;Piprazid;Piprazyl;Pipricide;Piptelate;Piverma;Polo-verm;Polyquil;Pripsen;Provtovermil;Razinol;Rondelim;Rondoxyl;Santoban;Siropar;Supraverm;Taenifigin;Teniver;Tivazine;Toxocan;Uricida;Uridina;Uroclear (hexamine);Urodan (phosphate);Urosolvina;Uvilon syrup (hydrate);Vanpar (hydrate);Veripar;Vermazine;Vermenter;Vermicompren;Vermidol;Vermifug;Vermilass;Vermipan;Vermiphsarmette;Vermiquimpe;Vermiquimyc;Vermisit;Vermitox;Vermofrik;Verocid;Wairmex;Wurmex;Wurmsirup siegfried
Multifuge;Multifuj;Nea-vermiol;Nemafugan;Nemasin;Nematocton;Nematorazine;Neo-ifusa;. | [World Health Organization (WHO)]
Piperazine was first used as a treatment for gout earlier this
century and its anthelminthic activity was discovered in 1949. It is also
considerably cheaper than other anthelminthic drugs. In some countries where
ascariasis is not endemic and where piperazine was used predominantly for the
treatment of pinworm it has been withdrawn from use on the grounds that other
more effective and less toxic drugs are now available (see full list). In other such
countries, however, piperazine remains available in over-the-counter preparations.
Clinical dosages occasionally induce transient neurological signs and concern has
been expressed that in some circumstances the drug may generate small amounts
of nitrosamine in the stomach. However, it is widely considered that these trace
doses are unlikely to give rise to a significant carcinogenic potential.
(Reference: (WHODIB) WHO Drug Information Bulletin, 1: 5, , 1983) | [Flammability and Explosibility]
Highlyflammable | [Pharmaceutical Applications]
A synthetic chemical, most commonly formulated as the citrate,
but also available as the adipate, edetate calcium and
tartrate salts. | [Mechanism of action]
Piperazine increases the resting potential
of the somatic musculature of nematodes, especially
in the syncytial region, by increasing the
permeability of the membrane to chloride ions.
This results in flaccid paralysis of the parasites,
which are expelled from the intestine . | [Pharmacokinetics]
Activity against intestinal worms requires that a substantial
amount remains in the gut. However, after oral administration
a variable amount is rapidly absorbed from the small
intestine and subsequently excreted in the urine. Its half-life
is extremely variable. | [Clinical Use]
Ascariasis
Pinworm | [Clinical Use]
Hexahydropyrazine or diethylenediamine (Arthriticine,Dispermin) occurs as colorless, volatile crystals of the hexahydratethat are freely soluble in water. After the discoveryof the anthelmintic properties of a derivative diethylcarbamazine,the activity of piperazine itself was established.Piperazine is still used as an anthelmintic for the treatmentof pinworm (Enterobius [Oxyuris] vermicularis) and roundworm(Ascaris lumbricoides) infestations. It is available invarious salt forms, including the citrate (official in the USP)in syrup and tablet forms. Piperazine blocks the response of the ascaris muscleto acetylcholine, causing flaccid paralysis in the worm,which is dislodged from the intestinal wall and expelled inthe feces. | [Synthesis]
Piperazine (38.1.12) is a bulk product in organic synthesis. It is made from
ethanolamine by heating it in ammonia at a temperature of 150¨C220??C and a pressure
of 100¨C250atm. It is used as a drug in the form of a salt, and as a rule, in the form of
adipinate.
| [Veterinary Drugs and Treatments]
Piperazine is used for the treatment of ascarids in dogs, cats, horses,
swine and poultry. Piperazine is considered safe to use in animals
with concurrent gastroenteritis and during pregnancy. | [Drug interactions]
Potentially hazardous interactions with other drugs
Pyrantel: antagonises effect of piperazine. | [Carcinogenicity]
No increase in lung adenomas
was produced in mice administered 0.69–18.75mg of piperazine/
kg in drinking water for 20–25 weeks and sacrificed
10–13 weeks later. Mice fed the equivalent of
938 mg/kg in the diet for 28 weeks and sacrificed at 40 weeks
failed to show any significant increase in the incidence of
lung adenomas. An increase in lung adenomas was
produced in this bioassay by administration of piperazine
together with sodium nitrate, suggesting the formation of the
active nitroso derivative. Sodium ascorbate inhibited
tumor formation, in theory, by preventing piperazine nitrosation
(304). Coadministration of 250 ppm piperazine and
500 ppm sodium nitrate in drinking water did not produce
tumors in rats. None of these studies were conducted
using currently accepted methods for evaluating carcinogenic
potential but piperazine alone, in these assays, was
noncarcinogenic. | [Environmental Fate]
This molecule has a simple chemical structure and molecular
weight of 86.14. It has a strong alkaline base soluble in water
(1:18), glycerol, and glycols, but is only sparingly soluble in
alcohol and insoluble in ether. Piperazine is not expected to
hydrolyze in water. The photodegradation half-life is approximately
0.8 h. The piperazine molecule is easily denaturalized
by diverse environmental factors and has a low potential for
bioaccumulation or biomagnification. To improve its stability,
it is usually formulated as different salts such as adipate, citrate,
phosphate, hexahydrate, and sulfate. Most piperazine salts are
white crystalline powders that are readily soluble in water.Exceptions are adipates, which dissolve to only a maximum
concentration of 5% in water, and phosphate, which is
insoluble. | [Metabolism]
About 25% is metabolised in the liver. Piperazine is
nitrosated to form N -mononitrosopiperazine (MNPz)
in gastric juice, which is then metabolised to N-nitroso-3-
hydroxypyrrolidine (NHPYR).
It is excreted in the urine mainly as metabolites. | [Purification Methods]
Piperazine crystallises from EtOH or anhydrous *benzene and is dried at 0.01mm. It can be sublimed under vacuum and purified by zone melting. The hydrochloride has m 172-174o (from EtOH), and the dihydrochloride crystallises from aqueous EtOH and has m 318-320o (dec, sublimes at 295-315o). The picrate has m ~200o, and the picrolonate crystallises from dimethylformamide ( m 259-261o). [Beilstein 23 H 4, 23 I 4, 23 II 3, 23 III/IV 15, 23/1 V 30.] | [Toxicity evaluation]
Piperazine blocks transmission by hyperpolarizing nerve
membranes at the neuromuscular junction, leading to parasite
immobilization by flaccid paralysis and consequent removal
from predilection and death. Piperazine is a selective agonist of
GABA receptors, resulting in the opening of chloride channels
and hyperpolarization of the membrane of the muscle cells of
nematode parasites. |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns.
R42/43:May cause sensitization by inhalation and skin contact .
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 2579 8/PG 3
| [WGK Germany ]
1
| [RTECS ]
TK7800000
| [F ]
3-8-23 | [Hazard Note ]
Harmful/Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29335900 | [Safety Profile]
Moderately toxic by
ingestion, skin contact, intravenous, and
subcutaneous routes. Mildly toxic by
inhalation. A skin and severe eye irritant.
Excessive absorption can cause urticaria,
vomiting, diarrhea, blurred vision, and
weakness. Combustible when exposed to
heat or flame; can react vigorously with
oxidizing materials. Explodes on contact
with dicyanofurazan. To fight fire, use
alcohol foam, mist, dry chemical, water
spray. When heated to decomposition it
emits highly toxic fumes of NOx. | [Hazardous Substances Data]
110-85-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 2600 mg/kg LD50 dermal Rabbit 8300 mg/kg |
| Questions And Answer | Back Directory | [Important pharmaceutical intermediates]
Piperazine is an important pharmaceutical intermediate, is mainly used for the production of anthelmintic piperazine phosphate, piperazine citrate and fluphenazine, strong pain, rifampicin, adipic acid piperazine, piperazine guanidine methyl tetracycline, quinoline piperazine phosphate, piperazine thiazole nitrate, enoxacin, hydroxyzine hydrochloride, trifluoperazine, diethylcarbamazine citrate, cinnarizine, flunarizine, decloxizine strong carbamazepine, prednisolone sodium phosphate, dexamethasone sodium phosphate, PPA, norfloxacin, ciprofloxacin, easy to cough piperazine, a piperazine Lee vancomycin, trimethoprim-triazine and other drugs. It is Also used for the production of surfactants products such as wetting agents, emulsifying agents,and dispersing agents ,and the production of plastic additives such as antioxidants, preservatives, stabilizers and rubber additives. It is derived from Dichloroethane by alcohol solution of ammonia.
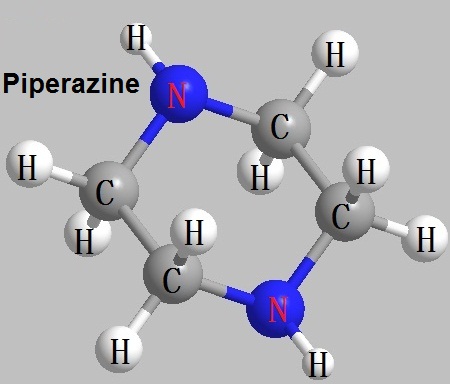
Figure 1 The structural formula of piperazine.
| [Pharmacology and mechanism of action]
Piperazine is a heterocyclic organic base widely used as an anthelminthic. It was originally developed for the treatment of gout. Its first successful use in helminthiasis was reported by Mouriquand et al. in 1951 [1]. Presently the drug is used in the treatment of infections caused by Ascaris lumbricoides and Enterobius vermicularis.
The drug causes flaccid paralysis in susceptible worms and the parasites lose their attachment to the intestinal wall, and are swept away by the normal bowel peristalsis. The biochemical mechanism behind this action is uncertain. Piperazine causes hyperpolarization of the Ascaris muscle rendering it unresponsive to acetylcholine [2].
| [Indications]
Treatment of infections due to Ascaris lumbricoides and Enterobius vermicularis. When cost and availability are not a consideration, safer and more effective drugs such as mebendazole or albendazole should be used instead.
| [Side effects]
Side effects commonly encountered with the recommended doses of piperazine are nausea, vomiting, abdominal cramps and diarrhoea which are usually mild and self-limiting. Although absolute incidence is unknown, severe side effects reported in the literature are rare. They can be classified into:
1. Allergic reactions such as urticaria, exantema, hypersensitivity, lacrimation, rhinorrea, productive cough, and bronchospasm[3,4].
2. Neuro-psychological reactions[5-11]:
(a) cerebral type such as vertigo, dizziness, tremor, incoordination, ataxia and hypotonia with EEG changes;
(b) psychic type such as depersonalization, hallucination and paranoic reactions;
(c) miscellaneous such as headache, visual disturbances, somnolence, coma and an increase in the number of petit mal attacks.
Neuro-psychological reactions are rare. Most cases reported concern children with pre disposing factors like neurological symptoms, renal diseases or those who have been treated with high doses of piperazine.
One case of haemolytic anaemia in a patient with G6PD deficiency [12], and one case of toxic hepatitis[13] have also been reported. However, no causal relationships can be established from these cases.
Nitrosation of piperazine to the potential carcinogen N-mononitrosopiperazine in the stomach of patients treated with normal therapeutic doses has been reported[14]. However, carcinogenicity related to the use of piperazine has not been reported despite the use of the drug over many years. In any case, this is unlikely to have any clinical implications with the short treatment period of nematodes.
| [Contraindications and precautions]
Piperazine should not be given to patients with hypersensitivity or with neurological diseases,especially epileptic patients.
| [Interactions]
In rats and mice, piperazine 1–5 g/kg subcutaneously, potentiates the side effects of chlorpromazine [15]. However, this is unlikely to have any clinical significance. Piperazine is antagonistic to pyrantel, bephenium and levamisole , but no potential clinical interactions have been reported.
| [Preparations]
Several preparations, apart from the one mentioned below, containing various piperazine salts are available.
• Antepar® (Wellcome). Oral suspension 150 mg piperazine hexahydrate/ml. Tablets 500 mg piperazine hexahydrate.
| [Acute oral toxicity]
rat LD50: 1900 mg/kg; Oral-Mouse LD50: 600 mg/kg
| [Data Skin irritation]
rabbit 500 mg Mild; Eyes-rabbit 0.25 mg/24 hours of severe
| [References]
1. Mouriquand G, Roman E, Coisnard J (1951). Essai de traitement de l’oxyurose par la piperazine. J Méd Lyon, 32, 189–195.
2. del Castillo J, De Mello WC, Morales T (1964). Mechanism of the paralysing action of piperazine on Ascaris muscle. Br J Pharmacol, 22, 463–477.
3. Macmillan AL (1973). Generalized pustular drug rash. Dermatologia, 146, 285–291.
4. McCullagh SF (1968). Allergenicity of piperazine: a study in environmental aetiology. Br J Ind Med, 25, 319–325.
5. Belloni C, Rizzoni G (1967). Neurotoxic side-effects of piperazine. Lancet, ii, 369.
6. Berger JR, Globus M, Melamed E (1979). Acute transitory cerebellar dysfunction associated with piperazine adipate. Arch Neurol, 36, 180–181.
7. Bomb RS, Bedi HK (1976). Neurotoxic side-effects of piperazine. Trans R Soc Trop Med Hyg, 70, 358.
8. Gupta SR (1976). Piperazine neurotoxicity and psychological reaction. J Ind Med Ass, 66, 33–34.
9. Parsons AC (1971). Piperazine neurotoxicity. ‘Worm wobble’. BMJ, 4, 790–792.
10. Vallat JN, Vallat JM, Texier J, Léger J (1972). Les signes neurologiques d’intoxication par la piperazine. Bordeaux Médicale, 5, 394–400.
11. Nickey LN (1966). Possible precipitation of petit mal seizures with piperazine citrate. J Am Med Ass, 195, 193–194.
12. Buchanan N, Cassel R, Jenkins T (1971). G-6-PD deficiency and piperazine. BMJ, 2, 110.
13. Hamlyn AN, Morris JS, Sarkany I, Sherlock S (1976). Piperazine hepatitis. Gastroenterology, 70, 1144–1147.
14. Bellander T, sterdahl B-G, Hagmar L (1985). Formation of N-mononitrosopiperazine in the stomach and its excretion in the urine after oral intake of piperazine. Toxicol Appl Pharmacol, 80, 193–198.
15. Sturman G (1973). Interaction between piperazine and chlorpromazine. Br J Pharmacol, 50, 153–155.
| [Flammability and hazardous characteristics]
Combustible; decomposition of toxic nitric oxide gas in case of thermal
| [Storage characteristics]
Treasury ventilation low-temperature drying; and stored separately from acid.
Since piperazine is corrosive, the flakes are stored in barrels lined with a polyethylene sack. To avoid yellowing, the barrels should be air tight and not exposed to direct sunlight. The aqueous solution is stored at 50 – 60 ℃ in insulated iron tanks that can be heated.
| [Extinguishing agent]
Water spray, dry powder, carbon dioxide, alcohol-resistant foam
| [Professional standards]
TWA 1 mg/m3; STEL 5 mg/m
|
|
|