| Identification | More | [Name]
Nalpha-Fmoc-Ndelta-Boc-L-ornithine | [CAS]
109425-55-0 | [Synonyms]
(2S)-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-5-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid
FMOC-L-ORN(BOC)
FMOC-L-ORN(BOC)-OH
FMOC-L-ORN(TBOC)-OH
FMOC-N-BOC-L-ORNITHINE
FMOC-(N-DELTA-BOC)-L-ORNITHINE
FMOC-ORN(BOC)-OH
FMOC-ORNITHINE(BOC)-OH
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-N-DELTA-T-BUTOXYCARBONYL-L-ORNITHINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-DELTA-TERT-BUTYLOXYCARBONYL-L-ORNITHINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-GAMMA-T-BUTYL-OXYCARBONYL-L-ORNITHINE
N-ALPHA-FMOC-N-DELTA-BOC-L-ORNITHINE
N-ALPHA-FMOC-N-DELTA-T-BOC-L-ORNITHINE
N-ALPHA-FMOC-N-DELTA-T-BUTYLOXYCARBONYL-L-ORNITHINE
N-ALPHA-FMOC-N-DELTA-TERT-BOC-L-ORNITHINE
N-DELTA-BOC-N-ALPHA-FMOC-L-ORNITHINE
RARECHEM EM WB 0176
N-tert-Butoxycarbonyl-N-9-fluorenylmethoxycarbonyl-L-ornithine
N-α-Boc-N-δ-Boc-L-ornithine
FMOC-ORN(BOC)-OH 98+% | [Molecular Formula]
C25H30N2O6 | [MDL Number]
MFCD00065668 | [Molecular Weight]
454.52 | [MOL File]
109425-55-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
111-115℃ | [Boiling point ]
679.0±55.0 °C(Predicted) | [density ]
1.226±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
soluble in Methanol | [form ]
powder to crystal | [pka]
3.85±0.21(Predicted) | [color ]
White to Almost white | [Detection Methods]
HPLC,NMR | [BRN ]
4772025 | [InChIKey]
JOOIZTMAHNLNHE-NRFANRHFSA-N | [SMILES]
C(O)(=O)[C@H](CCCNC(OC(C)(C)C)=O)NC(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O | [CAS DataBase Reference]
109425-55-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HazardClass ]
IRRITANT | [HS Code ]
29242990 |
| Hazard Information | Back Directory | [Description]
Fmoc-Orn(Boc)-OH is an ornithine-containing amino acid building block. It has been used to conjugate ornithine to GFP-labeled peptides, enhancing cell permeability when compared to unconjugated GFP-labeled peptides. This compound is useful in preparing cyclic peptides and special arginine derivates. | [Chemical Properties]
White powder | [Uses]
Fmoc-Orn(Boc)-OH is a biochemical reagent. It can be used as a biomaterial or organic compound in life science-related research. | [Preparation]
2 mmol L-Orn was weighted and dissolved in 15 ml of acetonitrile; 16 mmol Boc2O was dissolved in acetonitrile and added dropwise to an acetonitrile solution of ornithine, to react and generate a complex; 10 ml 20% sodium bicarbonate aqueous solution was added, and 2 g of anhydrous sodium carbonate and an appropriate amount of 8-hydroxyquinoline were added in batches; the mixture was stirred and reacted at room temperature for 4 hours to remove copper ions in the complex; then 2 mmol Fmoc-OSu was added to the aforesaid solution, followed by stirring at room temperature for 2 hours; the resultant was recrystallized with an ethyl acetate/petroleum ether mixed solvent, to obtain a peptide head intermediate Fmoc-Orn(Boc)-OH.
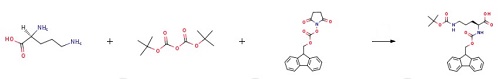 | [reaction suitability]
Reaction type: Fmoc solid-phase peptide synthesis | [Solubility in organics]
DMF: 20 mg/ml, DMF:PBS (pH 7.2) (1:5): 0.16 mg/ml, DMSO: 10 mg/ml, Ethanol: 10 mg/ml |
|
|