| Identification | Back Directory | [Name]
2-(chloromethyl)-4-methylquinazoline | [CAS]
109113-72-6 | [Synonyms]
Linaint-I
Linagliptin INT3
Linagliptin Impurity 67
Linagliptin interMediate A
CHLOROMETHYL)-4-METHYLQUINAZOLINE
2-chloroMethyl-4-Methyl quinaoline
2-(chloromethyl)-4-methylquizoline
Linagliptin 2-Chloromethyl Impurity
2-(chloromethyl)-4-methylquinazolin
2-(chloromethyl)-4-methylquinazoline
Quinazoline,2-(chloroMethyl)-4-Methyl-
2-(Chloromethyl)-4-methylquinazoline >
2-(Chloromethyl)-4-methylquinazoline (2-CMQ)
Linagliptin Impurity 35(Not Continued,see C4X-110132)
2-(chloromethyl)-4-methylquinazoline ISO 9001:2015 REACH
Linagliptin intermediates�����,2-(chloromethyl)-4-methylquinazoline
2-(Chloromethyl)-4-Methylquinazoline / Linagliptin Intermediate
Linagliptin intermediate Quinazoline, 2-(chloromethyl)-4-methyl-
(2-(chloromethyl)-4-methylquinazoline)����,2-(chloromethyl)-4-methylquinazoline
2-(chloromethyl)-4-methylquinazolineQ: What is
2-(chloromethyl)-4-methylquinazoline Q: What is the CAS Number of
2-(chloromethyl)-4-methylquinazoline Q: What is the storage condition of
2-(chloromethyl)-4-methylquinazoline | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C10H9ClN2 | [MDL Number]
MFCD09807547 | [MOL File]
109113-72-6.mol | [Molecular Weight]
192.645 |
| Chemical Properties | Back Directory | [Melting point ]
61.0 to 65.0 °C | [Boiling point ]
240.0±32.0 °C(Predicted) | [density ]
1.251 | [vapor pressure ]
0Pa at 20℃ | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
powder to crystal | [pka]
1.86±0.50(Predicted) | [color ]
Light yellow to Yellow to Orange | [Water Solubility ]
3.11g/L at 20℃ | [λmax]
318nm(EtOH)(lit.) | [InChIKey]
UHCUBOJGMLASBY-UHFFFAOYSA-N | [LogP]
1.9 at 25℃ |
| Hazard Information | Back Directory | [Chemical Properties]
White to yellow powder | [Uses]
2-(Chloromethyl)-4-methylquinazoline, is a building block used for the preparation of Linagliptin (L465900), and its impurities, acting as type 2 diabetes drugs. | [Synthesis Reference(s)]
Journal of Heterocyclic Chemistry, 23, p. 1263, 1986 DOI: 10.1002/jhet.5570230458 | [Flammability and Explosibility]
Notclassified | [Synthesis]
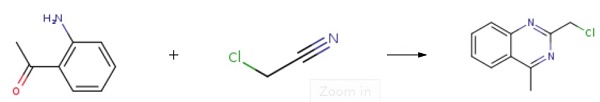
Into a 500ml reaction flask, add o-aminoacetophenone, chloroacetonitrile, 1,4-dioxane, and N-methyl-3(3-sulfopropyl)imidazolium hydrogen sulfate prepared above. Stir to dissolve, cool the reaction system to 8°C, and dropwise add hydrochloric acid to the reaction system. The reaction temperature was kept unchanged during the dropwise addition. After that, the reaction was continued at 8°C for 18 hours to complete the reaction process. After the reaction, ammonia water (the concentration of ammonia water was 10wt%) at 5°C was added to the reaction system. After stirring and crystallizing at 5 for 30 minutes, filter to obtain a filter cake, washing the filter cake with water. Recrystallized with 200ml of dichloromethane to obtain 2-(chloromethyl)-4-methylquinazoline. yield: 85.14%
 |
|
|