| Identification | Back Directory | [Name]
GSK1349572 sodiuM salt | [CAS]
1051375-19-9 | [Synonyms]
158439
GSK 1349572A
Dolutegravi Sodium
DOLUTEGRAVIR SODIUM
GSK1349572 sodiuM salt
Dolutegravir sodiuM salt
Dolutegravir SodiuM ( API)
Dolutegravir sodium(GSK1349572)
GSK1349572 sodiuM salt USP/EP/BP
Dolutegravir Sodium (GSK-1349572A)
(4R,12AS)-N-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyri
(4r,12as)-9-[(2,4-difluorophenyl)methylcarbamoyl]-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2h-pyrido[5,6]pyrazino[2,6-b][1,3]oxazin-7-olate
Sodium (4R,12aS)-9-((2,4-difluorobenzyl)carbamoyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate
(4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide sodium salt (1:1) | [EINECS(EC#)]
812-620-6 | [Molecular Formula]
C20H18F2N3NaO5 | [MDL Number]
MFCD28405599 | [MOL File]
1051375-19-9.mol | [Molecular Weight]
441.361 |
| Chemical Properties | Back Directory | [Melting point ]
>300oC | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly, Heated), Methanol (Slightly, Heated) | [form ]
Solid | [color ]
White to Green | [Stability:]
Hygroscopic | [InChIKey]
FWLDGCYHMZPGGI-SBBUJZKLNA-N | [SMILES]
O=C1N2[C@@H](CCO[C@@]2([H])CN2C=C(C(=O)NCC3C=CC(F)=CC=3F)C(=O)C(O)=C12)C.[NaH] |&1:3,7,r| |
| Hazard Information | Back Directory | [Description]
Dolutegravir sodium (1051375-19-9), developed and marketed by GlaxoSmithKline, was approved by the FDA in August 2013 as a novel integrase inhibitor for the treatment of HIV infection. Dolutegravir was fast-tracked by the FDA in February 2012, and joins an important class of drugs known as Integrase Strand Transfer inhibitors (INSTi’s). INSTi’s are characterized by their two-metal-chelating scaffolds, which are known to chelate Mg2+ cofactors in the enzyme active site, l interrupting function of HIV-1 integrase, which is essential for replication of viral DNA into host chromatin.Other drugs in this class, raltegravir and elvitegravir, are known to require either high dosages or PK boosting agents, respectively, with raltegravir also exhibiting substantial loss of potency in several major HIV-1 integrase mutation pathways. Dolutegravir was pursued with the goal of developing a novel INSTi with a once-daily, low-dosage treatment with improved resistance profile and without the need for the use of a PK boosting agent. Dolutegravir sodium has been approved for treating a broad population of HIV-infected patients, including adults undergoing their first treatment as well as those who have been treated with other integrase transfer strand inhibiting agents. | [Uses]
Dolutegravir is a second generation HIV-1 integrase strand transfer inhibitor. Dolutegravir is currently in Phase III clinical trials for the treatment of HIV infection. Dolutegravir has been shown to potently inhibit HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector. | [Definition]
ChEBI: Dolutegravir sodium is an organic sodium salt that is the monosodium salt of dolutegravir. Used for treatment of HIV-1. It has a role as a HIV-1 integrase inhibitor. It contains a dolutegravir(1-). | [Side effects]
Dolutegravir, an HIV medication, can lead to a variety of side effects. While some can be serious, many, such as nausea or sporadic dizziness, can be effectively managed. Dolutegravir may also cause alterations in your immune system, resulting in a condition known as immune reconstitution inflammatory syndrome (IRIS).
clinicalinfo.hiv.gov/en/drugs/dolutegravir/patient | [Synthesis]
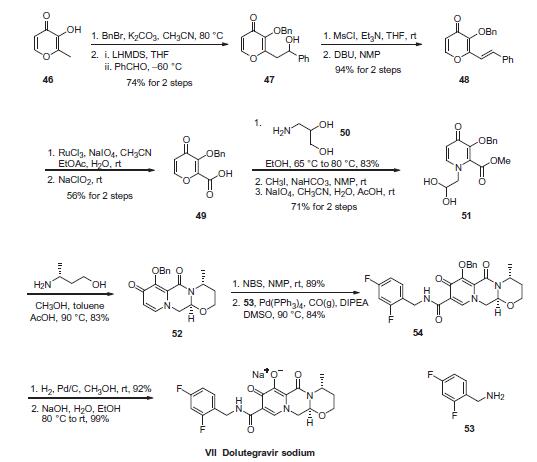
The process-scale synthesis of dolutegravir sodium is most likely to begin with the protection of pyrone 46 with benzyl and the alkylation of the resulting compound with benzaldehyde to produce alcohol 47 in 74% yield over two steps. Alcohol mesylation and in situ elimination provided the styrenyl olefin 48 in 94% yield, which further underwent an oxidative cleavage of the olefin to generate 49 by sequential addition of RuCl3/NaIO4 and NaClO2 (56% overall yield). Treatment of pyranone 49 with 3-amino-propane-2-diol (50) in ethanol at elevated temperatures delivered the corresponding pyridinone in 83% yield, and this was followed by esterification and sodium periodate-mediated diol cleavage to furnish intermediate 51 in 71% overall yield across the two-step sequence. l Next, the key ring-forming step in the synthesis of dolutegravir sodium consisted of cyclization of 51 with (R)-3- amino-butan-1-ol, a process which relies on substrate control to provide the desired tricyclic carbamoylpyridone system 52 in high stereoselectivity (20/1 in favor of the desired isomer).51 Previously, cyclization of systems such as 51 with unsubstituted amino alcohols were found to yield a mixture of diastereomeric products, therefore indicating the pivotal role of the chiral amino alcohol in influencing stereochemical bias during the overall cyclization step. In practice, reaction of 51 with (R)-3-amino-butan-1-ol at 90 led to isolation of a single cyclization product 52, after recrystallization from EtOAc. From 52, N-bromosuccinimide (NBS) bromination and subsequent treatment with amine 53 under palladium-catalyzed amidocarbonylative conditions led to amide 54 in 75% yield over 2 steps. Finally, removal of the benzyl group and subsequent crystallization using sodium hydroxide in water and ethanol provided dolutegravir sodium (VII) in 99% yield.
 | [in vitro]
gsk1349572 is a two-metal-binding hiv integrase strand transfer inhibitor whose mechanism of action was established through resistance passage experiments, integrase enzyme assays, mechanistic cellular assays and activity against viral strains resistant to other classes of anti-hiv agents. in a variety of cellular antiviral assays, gsk1349572 inhibited hiv replication with subnanomolar or low-nanomolar potency and with a selectivity index of 9,400. the protein-adjusted half-maximal effective concentration extrapolated to 100% human serum was 38 nm [1]. | [References]
[1] kobayashi m, yoshinaga t, seki t, wakasa-morimoto c, brown kw, ferris r, foster sa, hazen rj, miki s, suyama-kagitani a, kawauchi-miki s, taishi t, kawasuji t, johns ba, underwood mr, garvey ep, sato a, fujiwara t. in vitro antiretroviral properties of s/gsk1349572, a next-generation hiv integrase inhibitor. antimicrob agents chemother. 2011 feb;55(2):813-21.
[2] van lunzen j, maggiolo f, arribas jr, rakhmanova a, yeni p, young b, rockstroh jk, almond s, song i, brothers c, min s. once daily dolutegravir (s/gsk1349572) in combination therapy in antiretroviral-naive adults with hiv: planned interim 48 week results from spring-1, a dose-ranging, randomised, phase 2b trial. lancet infect dis. 2012 feb;12(2):111-8. |
|
|