| Identification | Back Directory | [Name]
Bis(4-biphenylyl)amine | [CAS]
102113-98-4 | [Synonyms]
2BP
IBBP
biphen-4-yl amine
Two(4-biphenyl)aMine
Bis(4-biphenyl)amine
4,4'-IMINOBIS(BIPHEN
Bis(4-biphenylyl)amin
di (4-biphenyl) amine
BIS(4-BIPHENYLYL)AMINE
Di(biphenyl-4-yl)amine
4,4'-IMINOBIS(BIPHENYL)
BIS-BIPHENYL-4-YL-AMINE
Bis(4-biphenylyl)amine >
Bis(4-biphenylyl)amine, BBA
N,N-Bis(4-phenylphenyl)amine
di([1,1`-biphenyl]-4-yl)aMine
4,4′-Bis[(4-broMophenyl)pheny
"N,N-Bis-(p-biphenylyl)amine "
N-(4-Biphenylyl)-4-biphenylamine
N-(Biphenyl-4-yl)biphenyl-4-amine
4-phenyl-N-(4-phenylphenyl)aniline
N-(Biphenyl-4-yl)biphenyl-4-amine 97%
Bis(4-biphenylyl)amine ISO 9001:2015 REACH
N-[1,1'-biphenyl]-4-yl-[1,1'-biphenyl]-4-Mine
[1,1'-Biphenyl]-4-aMine,N-[1,1'-biphenyl]-4-yl-
Bis(1,1'-biphenyl-4-yl)amine
Di(biphenyl-4-yl)amine | [EINECS(EC#)]
678-161-7 | [Molecular Formula]
C24H19N | [MDL Number]
MFCD08276279 | [MOL File]
102113-98-4.mol | [Molecular Weight]
321.41 |
| Chemical Properties | Back Directory | [Melting point ]
209 °C | [Boiling point ]
507.0±39.0 °C(Predicted) | [density ]
1.123±0.06 g/cm3(Predicted) | [vapor pressure ]
0-0Pa at 20-50℃ | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Acetonitrile (Slightly), Chloroform (Slightly), DMSO (Slightly) | [form ]
Solid | [pka]
0.67±0.40(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C24H19N/c1-3-7-19(8-4-1)21-11-15-23(16-12-21)25-24-17-13-22(14-18-24)20-9-5-2-6-10-20/h1-18,25H | [InChIKey]
JAUCIDPGGHZXRP-UHFFFAOYSA-N | [SMILES]
C1(C2=CC=CC=C2)=CC=C(NC2=CC=C(C3=CC=CC=C3)C=C2)C=C1 |
| Hazard Information | Back Directory | [Uses]
Bis(4-biphenylyl)amine can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly in laboratory research and development process and chemical production process. | [Synthesis]
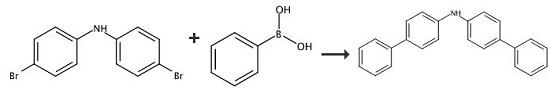
Bis(4-bromophenyl)amine (4.0 g, 12.3 mmol) and phenylboronic acid (4.0 g, 32.7 mmol) were mixed in 250 mL of toluene and 60 mL of ethanol. The solution was bubbled with nitrogen while stirring for 15 minutes. Pd(PPh3)4 (1.4 g, 1.23 mmol) and K3PO4 (13.5 g, 64 mmol) were added in sequence. The mixture was heated to reflux overnight under nitrogen. After cooling, the reaction mixture was filtered through filter paper and the solvent was then evaporated. The solid was redissolved in nitrogen-purged hot toluene and was filtered through a Celite?/silica pad when the solution was still hot. The solvent was then evaporated. The white crystalline solid was washed by hexane and air dried to obtain 3.8 g of Bis(4-biphenylyl)amine. |
|
|