| Identification | More | [Name]
Trenbolone | [CAS]
10161-33-8 | [Synonyms]
17BETA-HYDROXYESTRA-4,9,11-TRIEN-3-ONE
17-HYDROXYESTRA-4,9,11-TRIEN-3-ONE
4,9,11-ESTRATRIEN-17-BETA-OL-3-ONE
BETA-TRENBOLONE
TRENBOLONE
11-trien-3-one,17-beta-hydroxy-estra-9
17-beta-trenbolone
17-hydroxy-(17-beta)-estra-11-trien-3-one
ru2341
trenbolone--deascheduleiiiitem
trienbolone
TRENBOLONE:17-HYDROXYESTRA-4,9,11-TRIEN-3-ONE
TrenboloneEnanthateBase
trenbolon
Estra-4,9,11-trien-3-one, 17-beta-hydroxy
Estra-4,9,11-trien-3-one, 17-hydroxy-, (17-beta)-
17β-Trenbolone
R 2580
Trienolone
epi-Trebnbolone | [EINECS(EC#)]
600-229-1 | [Molecular Formula]
C18H22O2 | [MDL Number]
MFCD00214395 | [Molecular Weight]
270.37 | [MOL File]
10161-33-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
170°C | [alpha ]
D20 +19° (c = 0.45 in ethanol) | [Boiling point ]
490.8±45.0 °C(Predicted) | [density ]
1.19±0.1 g/cm3(Predicted) | [Fp ]
2℃ | [storage temp. ]
2-8°C
| [solubility ]
DMF: 30 mg/ml; DMF:PBS (pH 7.2)(1:3): 0.25 mg/ml; DMSO: 20 mg/ml; Ethanol: 2 mg/ml | [form ]
neat | [pka]
14.73±0.40(Predicted) | [InChI]
InChI=1/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h8-10,15-17,20H,2-7H2,1H3/t15-,16+,17+,18+/s3 | [InChIKey]
MEHHPFQKXOUFFV-OWSLCNJRSA-N | [SMILES]
[C@@]12([H])CC[C@H](O)[C@@]1(C)C=CC1=C3CCC(=O)C=C3CC[C@@]21[H] |&1:0,4,6,20,r| | [CAS DataBase Reference]
10161-33-8(CAS DataBase Reference) | [EPA Substance Registry System]
Estra-4,9,11-trien-3-one, 17-hydroxy-, (17.beta)- (10161-33-8) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R60:May impair fertility. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 1648 3 / PGII | [WGK Germany ]
3
| [RTECS ]
KG7752000
| [Safety Profile]
When heated to
decomposition it emits acrid smoke and
irritating fumes. |
| Hazard Information | Back Directory | [Description]
Trenbolone, also known as trienolone or trienbolone, is a kind of anabolic androgenic steroid belonging to the 19-nortestosterone group. It can be supplied to veterinary medicine of livestock in order the boost muscle growth and appetite. It can also enhance the performance of athletes. It has several physiological effects: (1) enhance protein synthesis and nitrogen retention in the muscle tissues, which boost enhanced anabolism; (2) greatly promote insulin-like growth factor-1 (IGF-1) which is a highly effective anabolic hormone; (3) Greatly increase the red blood cell count to boost blood oxygenation which leads to improved muscular endurance; (4) Inhibit glucocorticoid hormones which inhibit the growth of muscle tissue and promote fat. (5) Improve food efficiency. | [Chemical Properties]
Yellow Solid | [Originator]
Parabolan,Negma,France,1980 | [Uses]
Controlled substance (anabolic steroid). | [Definition]
ChEBI: Trenbolone is a 3-oxo-Delta(4) steroid that is estra-4,9,11-triene carrying an oxo group at position 3 and a hydroxy group at position 17beta. It is a synthetic anabolic steroid used for muscle growth in livestock. It has a role as a plant metabolite and an endocrine disruptor. It is a 3-oxo-Delta(4) steroid, a 17beta-hydroxy steroid, a C18-steroid and an anabolic androgenic steroid. | [Preparation]
Trenbolone synthesis: To a cold mixture of 47.9 g 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in 375 mL anhydrous methylene chloride (DCM), a solution of 54.9 g compound (II-a) and 11.5 mL acetic acid in 150 mL anhydrous DCM is added dropwise under stirring, while keeping the internal temperature at -5 °C. The addition funnel is rinsed with 25 mL DCM and this aliquot is added to the reaction mixture. After 4 hours, reaction conversion reaches 99.25% by HPLC. The reaction mixture is quenched by addition of a solution composed of 5.7 g of Na2S2O5, 50 mL water and 10 mL MeOH. The obtained slurry is warmed to 15-23°C and left stirring for 0.5 h, after which it was filtered. The filter cake is washed with 2x40 mL DCM and the solid discarded. The obtained biphasic mixture is separated, and the organic phase washed twice with a solution of 100 mL water, 10 mL MeOH and 3.9 g of NaHCO3. The phases are separated, and the organic layer washed one final time with a solution of 100 mL water, 10 mL MeOH and 3.9 g of NaHCO3. The combined solution is concentrated with stirring to 150 mL (total volume) under vacuum, keeping the internal temperature below 30 °C. To the resulting solution, 150 mL of acetone are added, and the obtained mixture concentrated under vacuum to 150 mL (total volume) again, keeping the internal temperature below 30 °C. This addition/concentration protocol is repeated 3 times. The resulting suspension is cooled to 0 °C and kept at this temperature for 0.5 h with stirring, after which it is filtered, and the filter cake is washed twice with 50 mL cold acetone. The wet solid is dried at 40 °C under vacuum to give Trenbolone in 76% yield and 98.2 % A/A purity by HPLC.
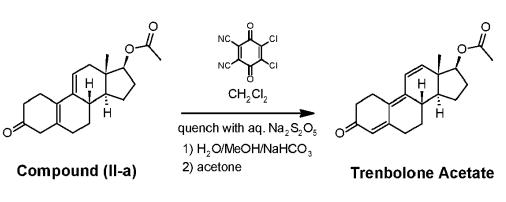
Synthesis of Trenbolone acetate | [Manufacturing Process]
Stage A: Preparation of 17β-Benzoyloxy-Estra-4,9,11-Trien-3-one - 0.400 g of
17β-benzoyloxy-4,5-seco-estra-9,11-diene-3,5-dione is dissolved in 4 cc of
toluene under an inert atmosphere. The solution is cooled to 3°C, then 0.48
cc is added of the solution of sodium tert-amylate in anhydrous toluene,
diluted by the addition of a further 4.8 cc of anhydrous toluene.
This reaction mixture is kept between 0°C and +5°C for six hours, with
agitation and under an inert atmosphere, then 5 cc of a 0.2 N solution of
acetic acid in toluene are added. The mixture is extracted with toluene, and
the extracts are washed with water and evaporated to dryness. The residue is
taken up in ethyl acetate, and then the solution is evaporated to dryness in
vacuo, yielding a resin which is dissolved in methylene chloride, and the
solution passed through a column of 40 g of magnesium silicate. Elution is
carried out first with methylene chloride, then with methylene chloride
containing 0.5% of acetone, and 0.361 g is thus recovered of a crude product,
which is dissolved in 1.5 cc of isopropyl ether; then hot methanol is added
and the mixture left at 0°C for one night.
0.324 g of the desired 17β-benzoyloxy-estra-4,9,11-trien-3-one are thus
finally obtained, being a yield of 85%, melting point is 154°C.
Stage B: Preparation of 17β-Hydroxy-Estra-4,9,11-Trien-3-one - 3 g of 17β-
benzoyloxy-estra-4,9,11-trien-3-one, obtained as described in Stage A are
dissolved in 15 cc of methanol. 0.03 g of hydroquinone is added, and the
mixture is taken to reflux while bubbling in nitrogen. Then 1.2 cc of 11%
methanolic caustic potash is added and reflux is maintained for three hours,
after which the reaction product is acidified with 0.36 cc of acetic acid.
The methyl benzoate thus formed is eliminated by steam distillation, and
2.140 g of crude product are obtained, which are dissolved in 20 cc of
methylene chloride. This solution is passed through 10 parts of magnesium
silicate, elution being performed with 250 cc of methylene chloride containing
5% of acetone. After evaporation of the solvent 2.050 g of product is
recovered, which is recrystallized from isopropyl ether.
In this way 1.930 g of the desired 17β-hydroxy-estra-4,9,11-trien-3-one is
obtained being a yield of 89%, melting point is 186°C. It is converted to the
acetate by acetic anhydride. | [Brand name]
[Trenbolone is INN and BAN. | [Therapeutic Function]
Anabolic steroid | [Side effects]
Trenbolone's side effects aren't only physical but also mental, with users commonly reporting feeling increasingly: irritable, anxious, paranoid and depressed (than on other steroids).
Such side effects can be linked to Trenbolone having a stimulating effect on the central nervous system, causing an increase in adrenaline output and thus shifting Tren-users into a state of fight or flight mode. | [References]
https://en.wikipedia.org/wiki/Trenbolone
https://www.steroid.com/Trenbolone.php
Škorjanc, D, M. Brus, and I. Vojtic. "A short review of chain controlling systems in livestock production technology. " Agricultura (2005). |
|
|