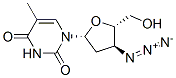
Zidovudine synthesis
- Product Name:Zidovudine
- CAS Number:30516-87-1
- Molecular formula:C10H13N5O5
- Molecular Weight:283.24

50-89-5
634 suppliers
$9.00/1g

30516-87-1
489 suppliers
$13.50/1G
Yield:30516-87-1 75 g
Reaction Conditions:
Stage #1:thymidine with thionyl chloride in 1-methyl-pyrrolidin-2-one at 5 - 10;
Stage #2: with sodium hydrogencarbonate in 1-methyl-pyrrolidin-2-one at 110;
Stage #3: with lithium azide in 1-methyl-pyrrolidin-2-one at 135;Reagent/catalyst;
Steps:
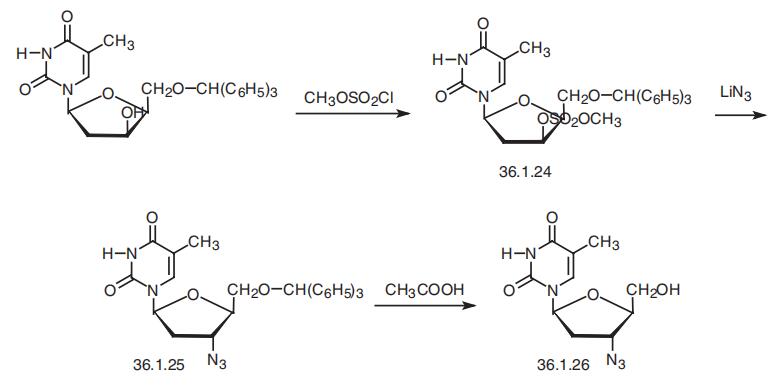
1-8 Example 4 Preparation of Zidovudine
The preparation method of zidovudine (the preparation process thereof is shown in Fig. 8) includes the following steps:1) 100 g of thymidine was suspended in 300 ml of N-methylpyrrolidone, 50 g of thionyl chloride was added dropwise at 5 to 10 ° C under stirring, and the reaction was completed by HPLC to obtain a reaction liquid;2) adding 110 g of sodium hydrogencarbonate to the reaction liquid prepared in the step 1), raising the temperature to 110 ° C, and stirring under the conditions of HPLC, until the reaction is completed, to obtain a reaction liquid;3) 42 g of lithium azide was added to the reaction liquid of the step 2), and the temperature was raised to 135 ° C, and the reaction was completed by HPLC under stirring to obtain a reaction liquid containing zidovudine. Example 5-8 purification of zidovudine Example 5-8 zidovudine purification process purified (refined purified condition results in Table 1), comprising the steps of: 1) The reaction solution containing zidovudine is cooled to 70-80 ° C, and N-methylpyrrolidone is recovered by distillation under reduced pressure. The residue is diluted with 200 ml of water and extracted with ethyl acetate or isopropyl acetate three times. The secondary dosage is 300 ml; after three times of extracting the organic phase, 3 g of activated carbon is added, the mixture is decolorized by stirring, the activated carbon is removed by filtration under reduced pressure, The collected filtrate is distilled under reduced pressure to obtain a crude zidovudine; 2) The zidovudine crude product was recrystallized from hot water to obtain a zidovudine refined product, which was a white solid.
References:
Anhui Baker Joint Pharmaceutical Co., Ltd.;Yue Xiangjun;Zhong Xiaofeng;Zou Chunwei;Jiang Jiwang;Chen Xiaofeng;Wang Zhibang CN104710490, 2019, B Location in patent:Paragraph 0045-0071
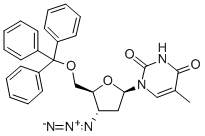
29706-84-1
76 suppliers
inquiry

30516-87-1
489 suppliers
$13.50/1G
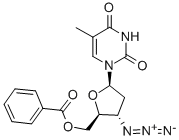
106060-78-0
13 suppliers
inquiry

30516-87-1
489 suppliers
$13.50/1G
![1-[(2R,4S,5S)-4-Amino-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione](/CAS/GIF/52450-18-7.gif)
52450-18-7
86 suppliers
$60.10/1g

30516-87-1
489 suppliers
$13.50/1G
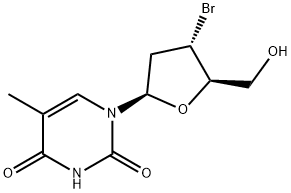
99785-51-0
7 suppliers
inquiry

30516-87-1
489 suppliers
$13.50/1G
