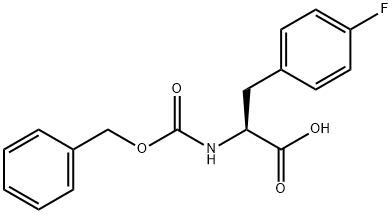
Z-P-FLUORO-PHE-OH synthesis
- Product Name:Z-P-FLUORO-PHE-OH
- CAS Number:17543-58-7
- Molecular formula:C17H16FNO4
- Molecular Weight:317.31

1132-68-9
265 suppliers
$12.50/1G

501-53-1
398 suppliers
$10.00/1g

17543-58-7
79 suppliers
$20.00/1G
Yield:17543-58-7 95%
Reaction Conditions:
with sodium hydrogencarbonate;potassium carbonate in tetrahydrofuran;water at 20;
Steps:
(S)-2-(((Benzyloxy)carbonyl)amino)-3-(4-fluorophenyl)propanoic acid (31)
To a solution of ^-fluoro-L-phenylalanine (2.56 g, 13.98 mmol), NaHC0 (1.76 g, 21 mmol), K2C03 (2.90 g, 21 mmol) in THF-H20 (v/v=l : l, 50 mL) was added CbzCl (2.2 mL, 15.4 mmol). The reaction mixture was stirred overnight at room temperature. After evaporation of the volatils , the reaction mixture was washed with ethyl acetate (10 mL) and then the pH of the water phase was adjusted to pH =1 by addition of IN HC1. The water layer was finally extracted with ethyl acetate (30 mL x 4) and the combined organic layers dried over Na2S04. to give, after evaporation, compound 31 (4.2 g, 95%). 1H NMR (400 MHz, Methanol-^) δ 7.37 - 7.12 (m, 7H), 6.96 (t, J = 8.8 Hz, 2H), 5.11 - 4.93 (m, 2H), 4.47 (dd, J = 9.3, 5.0 Hz, 1H), 3.19 (dd, J = 14.0, 5.0 Hz, 1H), 2.92 (dd, J = 14.0, 9.3 Hz, 1H). 1 C NMR (101 MHz, MeOD) δ 173.61, 163.03, 160.62, 156.94, 136.74, 133.08, 133.05, 130.72, 130.64, 128.08, 127.61, 127.34, 114.78, 114.57, 66.20, 55.37, 36.45. 19F NMR (377 MHz, Methanol-^) δ - 119.46 . LC-MS: m/z [M+H]+ calcd. for Ci7Hi7FN04: 318.1, found: 318.2.
References:
WO2017/197377,2017,A1 Location in patent:Page/Page column 66
![3-Oxazolidinecarboxylic acid, 4-[(4-fluorophenyl)methyl]-2,5-dioxo-, phenylmethyl ester](/CAS/20210305/GIF/394210-36-7.gif)
394210-36-7
0 suppliers
inquiry
117467-73-9
30 suppliers
$56.89/250MG

17543-58-7
79 suppliers
$20.00/1G

51-65-0
201 suppliers
$12.50/1G

17543-58-7
79 suppliers
$20.00/1G
3005-66-1
18 suppliers
$70.00/1g

17543-58-7
79 suppliers
$20.00/1G
13139-17-8
504 suppliers
$6.00/25g

17543-58-7
79 suppliers
$20.00/1G