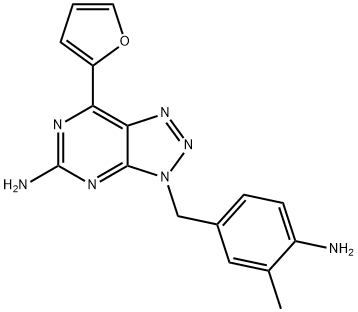
Vipadenant synthesis
- Product Name:Vipadenant
- CAS Number:442908-10-3
- Molecular formula:C16H15N7O
- Molecular Weight:321.34
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

442908-10-3
69 suppliers
$55.00/500μg
Yield: 92%
Reaction Conditions:
with formic acid;triethylamine;5% palladium over charcoal in tetrahydrofuran at 70; for 5 h;
Steps:
4
Example 4: Preparation of3-(4-amino-3-methylbenzyl)-7-(furan-2-yl)-3H- [l,2,3]triazolo[4,5-d]pyrimidin-5-amine (1) A 250 mL 2-necked round-bottomed flask was charged with 7-(furan-2-yl)-3-(3- methyl-4-nitrobenzyl)-3H-[l,2,3]triazolo[4,5-d]pyrimidin-5-amine (3.0 g, 8.5 mmol) and 5% Pd/C catalyst (0.46 g, 0.073 mmol) under a nitrogen atmosphere. Next, THF (72 mL) and triethylamine (9.0 mL, 65 mmol) were added via syringe and the resulting mixture was stirred to obtain slurry. Formic acid (2.3 mL, 46.03 mmol) was then added all at once, and the mixture was heated with a bath set to a temperature of 70 0C. After 5 hours the reaction was cooled to 25 0C. Water (60 mL) was added, and concentrated hydrochloric acid was added dropwise to dissolve the product. The solution was filtered through Celite 545 to remove the catalyst, and the filter cake was washed with additional water (2 X 5 mL). To the yellowish filtrate was added 50% sodium hydroxide in water to precipitate the product. The mixture was stirred for an additional hour before isolation by filtration. The filter cake was washed with water (10 mL) then methanol (10 mL). The product was dried under vacuum to constant weight to yield 2.79 g (92%) of 3-(4-amino- 3-methylbenzyl)-7-(furan-2-yl)-3H-[l,2,3]triazolo[4,5-d]pyrimidin-5-amine.
References:
BIOGEN IDEC MA INC.;VERNALIS RESEARCH LIMITED WO2008/86201, 2008, A1 Location in patent:Page/Page column 21
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
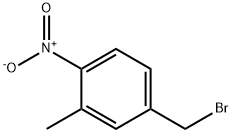
141281-38-1
49 suppliers
$116.64/1gm:

442908-10-3
69 suppliers
$55.00/500μg