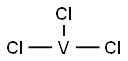
Vanadium(III) chloride synthesis
- Product Name:Vanadium(III) chloride
- CAS Number:7718-98-1
- Molecular formula:Cl3V
- Molecular Weight:157.29
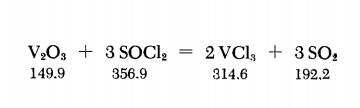
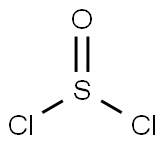
7719-09-7
406 suppliers
$15.00/10g
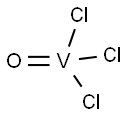
7727-18-6
61 suppliers
$12.30/10g
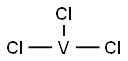
7718-98-1
90 suppliers
$20.00/1g
Yield:7718-98-1 90%
Reaction Conditions:
with N2H5Cl in tetrachloromethaneheating 10 - 15 h under N2 at reflux;; heating with CS2; pptn. washing with CHCl3 and ether;;
References:
Seifert, H. J. [Zeitschrift fur Anorganische und Allgemeine Chemie][Zeitschrift fuer Anorganische und Allgemeine Chemie,1962,vol. 317,p. 123 - 128]
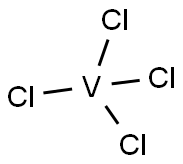
7632-51-1
46 suppliers
$758.57/1G
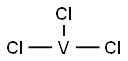
7718-98-1
90 suppliers
$20.00/1g
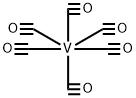
14024-00-1
4 suppliers
inquiry
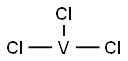
7718-98-1
90 suppliers
$20.00/1g

7440-62-2
178 suppliers
$38.00/5g

7782-50-5
2 suppliers
$373.00/454g
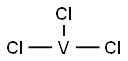
7718-98-1
90 suppliers
$20.00/1g

7727-18-6
61 suppliers
$12.30/10g
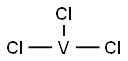
7718-98-1
90 suppliers
$20.00/1g