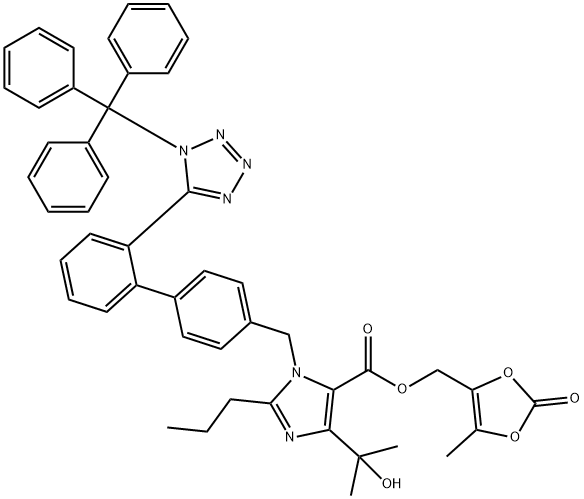
Trityl olmesartan synthesis
- Product Name:Trityl olmesartan
- CAS Number:144690-92-6
- Molecular formula:C48H44N6O6
- Molecular Weight:800.9
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
135 suppliers
inquiry
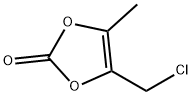
80841-78-7
530 suppliers
$10.00/250mg

144690-92-6
270 suppliers
$9.00/1g
Yield:144690-92-6 96.5%
Reaction Conditions:
Stage #1:ethyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-({2'-[1-(triphenylmethyl)-1H-1,2,3,4-tetrazol-5-yl]-[1,1'-biphenyl]-4-yl}methyl)-1H-imidazole-5-carboxylate with lithium hydroxide monohydrate in tetrahydrofuran at 20; for 18 h;Large scale;
Stage #2:4-chloromethyl-5-methyl-1,3-dioxol-2-one with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 20 - 40; for 2 h;Large scale;
Steps:
2 Example 2
188.0kg of tetrahydrofuran was added to a glass-lined reactor, 54.0kg 4- (1- hydroxy-1-methylethyl) -2-propyl-1- {4- [2- (trityl-tetrazol -5 - yl) phenyl] phenyl} methylimidazole-5-carboxylate, 92.0.0kg 4.0kg purified water and lithium hydroxide monohydrate.After complete addition, 20 stirred for 18 hours, once every 2 hours the reaction temperature recording, tracking and detection TLC starting material to substantially complete the reaction.After completion of the reaction, 240.0kg Ethyl acetate and sodium chloride solution (20.0 kg of sodium chloride was added to 180.0kg purified water, and dissolved with stirring), stirred for 10 minutes, allowed to stand for 20 minutes stratification.The organic phase was washed with sodium chloride solution (32.0 kg of sodium chloride was added to 288.0kg purified water, stirred and dissolved) Average washed twice, each wash was stirred for 10 minutes and allowed to stand for 20 minutes.The organic phase was collected, dried over anhydrous sodium sulfate and stirred for 4 hours.Filtered, and the filtrate was concentrated under reduced pressure to remove ethyl acetate (water bath temperature controlled at 45 ± 5 , the degree of vacuum at -0.1 ~ -0.06MPa), a solution 188.0kg N, N- dimethylformamide, is added 15.3kg anhydrous potassium carbonate, 2.7kg of potassium iodide was stirred at 20 ± 10 was slowly added 14.5kg 4- chloro-5-methyl-1,3-dioxol-2-one, addition was completed, heating, feed temperature control reaction was stirred for 2 hours at 40 ± 2 .After completion of the reaction, the material was cooled to 15 ± 5 rejection filter, and the filtrate was added to a solution of sodium chloride (338.0kg sodium chloride to 940.0kg purified water, and dissolved with stirring), the following -5 ~ 0 stirred for 4h, rejection was filtered, the filter cake was charged to an oven baking pan blast drying oven at a temperature of 40 ± 5 8 hours.To give 58.2kg of trityl olmesartan medoxomil, a yield of 96.5%.
References:
Lianyungang Runzhong Pharmaceutical Co., Ltd.;Zhong Zhaobo;Cheng Jinrong;He Shaojie;Tang Zhaocheng CN110396084, 2019, A Location in patent:Paragraph 0030-0033
879097-59-3
1 suppliers
inquiry

80841-78-7
530 suppliers
$10.00/250mg

144690-92-6
270 suppliers
$9.00/1g
761404-85-7
100 suppliers
inquiry

80841-78-7
530 suppliers
$10.00/250mg

144690-92-6
270 suppliers
$9.00/1g
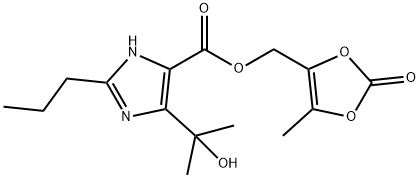
144978-05-2
20 suppliers
inquiry
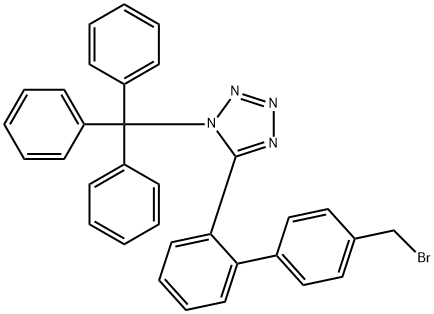
124750-51-2
457 suppliers
$6.00/1g

144690-92-6
270 suppliers
$9.00/1g
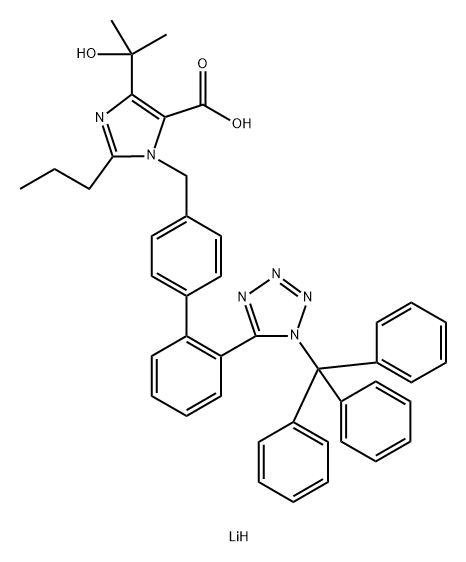
1114761-92-0
5 suppliers
inquiry

80841-78-7
530 suppliers
$10.00/250mg

144690-92-6
270 suppliers
$9.00/1g