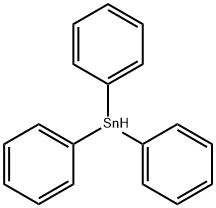
TRIPHENYLTIN HYDRIDE synthesis
- Product Name:TRIPHENYLTIN HYDRIDE
- CAS Number:892-20-6
- Molecular formula:C18H16Sn
- Molecular Weight:351.03
58-22-0
410 suppliers
$19.00/1 mg
639-58-7
190 suppliers
$20.00/10 g

892-20-6
49 suppliers
$75.59/5g
Yield:-
Reaction Conditions:
in ethanol
Steps:
3 TRIPHENYLTIN TESTOSTERONYL ETHER
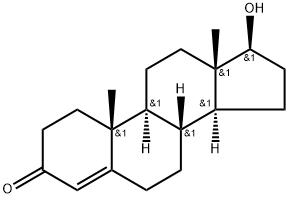
EXAMPLE 3 TRIPHENYLTIN TESTOSTERONYL ETHER 3.5 g of triphenyltin chloride was dissolved in 40 ml of ethanol and 2.8 g of testosterone was dissolved in 40 ml of ethanol. Both solutions were mixed in a 200 ml round bottom flask fitted with a soxhlet and a reflux condenser. The mixture was refluxed for 3 hours at 78°-80° C. The reaction product duct was filtered and evaporated to 1/4 its volume, then cooled to form a powder. Appearance: amorphous powder Melting point: 75°-80° C. MW (determined by mass spec): 720
References:
Cardarelli;Nathan F.;Kanakkanatt;Sebastian V. US4634693, 1987, A
16853-85-3
416 suppliers
$14.00/1g

639-58-7
190 suppliers
$20.00/10 g

892-20-6
49 suppliers
$75.59/5g

639-58-7
190 suppliers
$20.00/10 g

892-20-6
49 suppliers
$75.59/5g
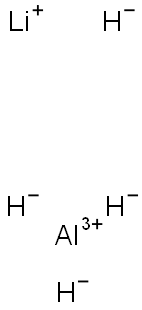
16853-85-3
416 suppliers
$14.00/1g

894-09-7
15 suppliers
$144.00/1ea

892-20-6
49 suppliers
$75.59/5g
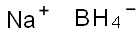
16940-66-2
8 suppliers
$19.19/100ml

639-58-7
190 suppliers
$20.00/10 g

892-20-6
49 suppliers
$75.59/5g