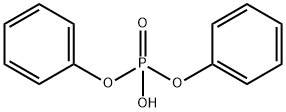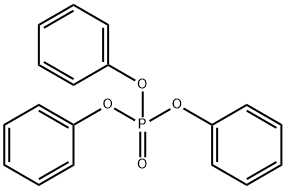
Triphenyl phosphate synthesis
- Product Name:Triphenyl phosphate
- CAS Number:115-86-6
- Molecular formula:C18H15O4P
- Molecular Weight:326.28
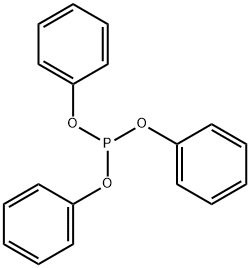
101-02-0
385 suppliers
$10.00/5g

115-86-6
551 suppliers
$6.00/25g
Yield:115-86-6 99%
Reaction Conditions:
with 1-methyl-3-(4-((2,4,6-triisopropylphenyl)tellanyl)benzyl)-1H-imidazol-3-ium hexafluorophosphate;Rose Bengal lactone at 15; for 2.5 h;Irradiation;Ionic liquid;Reagent/catalyst;
Steps:
Triphenyl Phosphate (Table 2, Entry 1); Typical Procedure
General procedure: A solution of (PhO)3P (0.0773 g, 0.250 mmol) in (bmim)[PF6] (5mL) containing 5 (0.0330 g, 0.0500 mmol) and rose bengal(0.0128 g, 0.0125 mmol) was vigorously stirred in an open flaskand irradiated with a 60 W LED lamp for 2.5 h. The temperaturewas kept at about 15 °C by using an ice bath during the irradiation.The resulting mixture was extracted with Et2O, and thesolvent was then evaporated to give a pink solid; yield: 0.0803 g(99%); mp 44-47 °C.1H NMR (500 MHz, CDCl3): = 7.36 (t, J = 7.7, 6 H), 7.25-7.19(m, 9 H). 13C NMR (125 MHz, CDCl3): = 150.6, 150.5, 130.0,125.7, 120.3, 120.2. 31P NMR (202 MHz, CDCl3): = -17.7. HRMS(APCI): m/z [M + H]+ calcd for C18H16O4P: 327.0781; found:327.0743.
References:
Mihoya, Aya;Shibuya, Yuga;Ito, Akane;Toyoda, Anna;Oba, Makoto;Koguchi, Shinichi [Synlett,2020,vol. 31,# 20,p. 2043 - 2045] Location in patent:supporting information
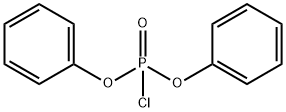
2524-64-3
391 suppliers
$14.00/5g
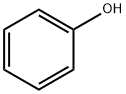
108-95-2
743 suppliers
$14.00/25g

115-86-6
551 suppliers
$6.00/25g

108-95-2
743 suppliers
$14.00/25g

115-86-6
551 suppliers
$6.00/25g
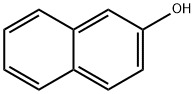
135-19-3
742 suppliers
$9.00/1g

108-95-2
743 suppliers
$14.00/25g

115-86-6
551 suppliers
$6.00/25g
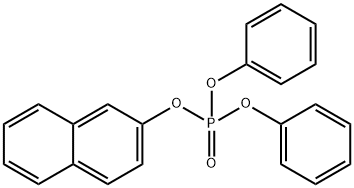
18872-49-6
45 suppliers
$6.00/250mg
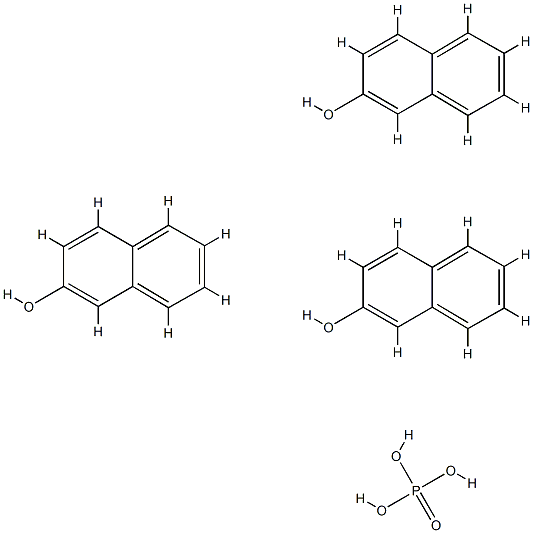
7657-86-5
3 suppliers
inquiry
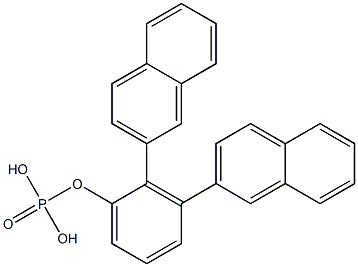
18872-50-9
1 suppliers
inquiry