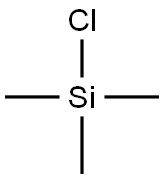
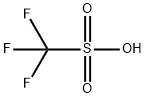
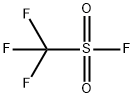
Trimethylsilyl trifluoromethanesulfonate synthesis
- Product Name:Trimethylsilyl trifluoromethanesulfonate
- CAS Number:27607-77-8
- Molecular formula:C4H9F3O3SSi
- Molecular Weight:222.26
A kind of reaction is to prepare with tetramethylsilane by trifluoromethanesulfonic acid. However, tetramethylsilane in the preparation process often with a large amount of dimethylbutanes, dimethylchlorosilane, impurities such as dimethyl dichlorosilane (DMCS), above-mentioned impurity and trifluoromethanesulfonic acid is easy to occur side reaction, especially the trifluoromethanesulfonic acid dimethyl silicone grease that dimethylchlorosilane and trifluoromethanesulfonic acid generate and the boiling point of trimethylsilyl triflate are very approaching, be difficult to separate by the method for rectifying.
Another kind of preparation method is fluoroform sulphonate and silylation reagent reaction. This preparation method can obtain a purity of up to 99.9% Trimethylsilyl trifluoromethanesulfonate.
Yield:27607-77-8 96.55%
Reaction Conditions:
in n-heptane at 50 - 60; for 13 h;Large scale;
Steps:
1-3 Example 3
Take 26kg of trimethylchlorosilane and 14kg of n-heptane and add them to a 100L reaction flask.Take 35kg of trifluoromethanesulfonic acid, slowly drip into the reaction kettle, control the internal temperature of 50-60 (hydrochloric acid gas is generated, water is absorbed, and dilute hydrochloric acid is made by yourself), and it takes about 10h.After dripping, the reaction is kept for 3h.Concentrate under reduced pressure (recover n-heptane), and rectify the concentrated liquid with a water pump to obtain 50 kg of product with a yield of 96.55% and a purity of 98%
References:
CN112707927,2021,A Location in patent:Paragraph 0018-0023
1066-40-6
223 suppliers
$14.00/1g
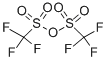
358-23-6
591 suppliers
$10.00/1g

27607-77-8
504 suppliers
$10.00/5g
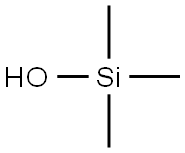
1066-40-6
223 suppliers
$14.00/1g
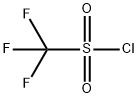
421-83-0
242 suppliers
$22.39/1G

27607-77-8
504 suppliers
$10.00/5g

107-46-0
465 suppliers
$10.00/10g

27607-77-8
504 suppliers
$10.00/5g