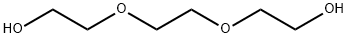
Triethylene glycol synthesis
- Product Name:Triethylene glycol
- CAS Number:112-27-6
- Molecular formula:C6H14O4
- Molecular Weight:150.17
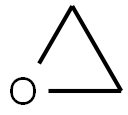
75-21-8
225 suppliers
$39.10/1mL

111-46-6
1073 suppliers
$5.00/25g
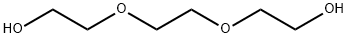
112-27-6
753 suppliers
$6.00/100g
Yield:-
Reaction Conditions:
with titanium(IV) oxide in tetrahydrofuran at 120; under 2250.23 Torr;Inert atmosphere;
Steps:
2.3. Reaction procedure for alkoxylations with solid catalyst
General procedure: The corresponding nucleophile (water or alcohol, typically 0.5mmol), 1.8 ml of ethylene oxide in THF (6 mmol, 3.3 M) and 65 mg of solid catalyst (25 wt%) were placed in a double-walled 6 mL vial, equipped with a magnetic stirrer. The reactor was closed with a screw cap connected to a manometer. The reactor was purged three times withN2 and finally charged with 3 bars of N2. The reaction mixture wasplaced in a pre-heated oil bath at 120 °C and magnetically stirred over 16 h. At the end of the reaction, the mixture was cooled, filtered,concentrated and analyzed by 1H-NMR and GC-MS.
References:
Ballesteros–Soberanas, Jordi;Leyva–Pérez, Antonio;Martínez–Castelló, Aarón;Oliver–Meseguer, Judit;Tejeda–Serrano, María [Molecular catalysis,2021,vol. 515,art. no. 111927]

75-21-8
225 suppliers
$39.10/1mL

107-21-1
1329 suppliers
$10.00/25g

111-46-6
1073 suppliers
$5.00/25g
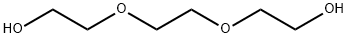
112-27-6
753 suppliers
$6.00/100g

2615-15-8
225 suppliers
$6.00/1g

64-18-6
1075 suppliers
$22.12/250ML
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
347 suppliers
$7.00/25g
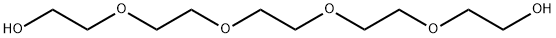
4792-15-8
252 suppliers
$6.00/1g

107-21-1
1329 suppliers
$10.00/25g
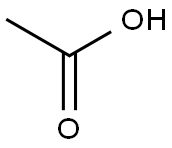
64-19-7
1551 suppliers
$10.00/25ML

111-46-6
1073 suppliers
$5.00/25g
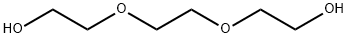
112-27-6
753 suppliers
$6.00/100g

75-21-8
225 suppliers
$39.10/1mL
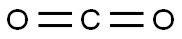
124-38-9
127 suppliers
$214.00/14L

96-49-1
496 suppliers
$6.00/100g

107-21-1
1329 suppliers
$10.00/25g

111-46-6
1073 suppliers
$5.00/25g
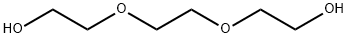
112-27-6
753 suppliers
$6.00/100g
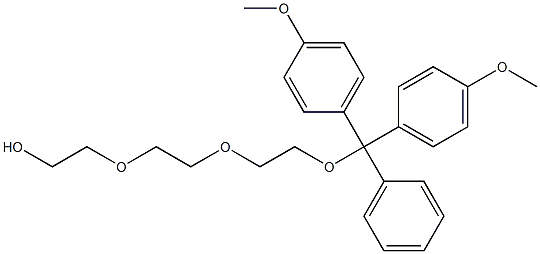
146669-11-6
7 suppliers
$500.83/5MG
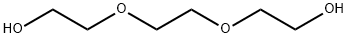
112-27-6
753 suppliers
$6.00/100g