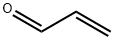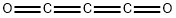
tricarbon dioxide synthesis
- Product Name:tricarbon dioxide
- CAS Number:504-64-3
- Molecular formula:C3O2
- Molecular Weight:68.03
CH2(COOH)2→ C3O2 + 2H2O
Alternatively, the compound may be prepared by thermal dissociation of diacetyltartaric anhydride.
Yield:-
Reaction Conditions:
with oxygen-precovered Au(110) catalyst at 169.84;
Steps:
6
Methanol, acrolein and methacrolein each show characteristic reaction patterns with preadsorbed atomic oxygen. As discussed, methanol self-couples to form methyl formate at 250 K via adsorbed methoxy intermediate (Xu, B. et al. Agnew. Chem. Int. Ed. 2009, 48, 4206; Stowers, K. J. et al. J. Catal. 2013, 308, 131 ; Personick, M. L. et al. ACS Catal. 2015, 5, 4237). The reaction of acrolein with oxygen- precovered Au(1 1 0) yields, characteristically, carbon suboxide (m/z = 68) at 443 K with minor amounts of acrylic acid (m/z = 72) (Fig. 20a). Acrolein (m/z = 56) desorbing below 300 K corresponds to unreacted aldehyde. There is some combustion to carbon dioxide (m/z = 44), due to over-oxidation under the conditions employed and production of water (m/z = 18) below 400 K. Methacrolein is oxidized to methacrylic acid at 300-450 K as well as CO2 at 350 K (Fig. 20c). When coadsorbed at 120 K on Au(1 10) precovered with 0.1 ML of adsorbed O, methanol and acrolein (methacrolein) cross-couple to form methacrylate and methyl methacrylate at 280 and 320 K, respectively (Fig. 20b, d). Similar results were observed on np(Ag)Au for acrolein in ultrahigh vacuum.
References:
PRESIDENT AND FELLOWS OF HARVARD COLLEGE;LAWRENCE LIVERMORE NATIONAL LABORATORY;FRIEND, Cynthia, M.;MADIX, Robert, J.;ZUGIC, Branko;WANG, Lucun;PERSONICK, Michelle, L.;BIENER, Juergen;BIENER, Monika, Margarete WO2017/127728, 2017, A1 Location in patent:Page/Page column 27
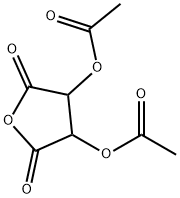
122376-19-6
6 suppliers
inquiry
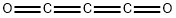
504-64-3
4 suppliers
inquiry
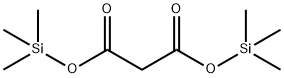
18457-04-0
108 suppliers
$10.00/1g
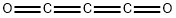
504-64-3
4 suppliers
inquiry
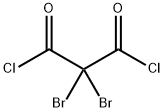
74539-43-8
0 suppliers
inquiry
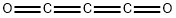
504-64-3
4 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
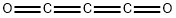
504-64-3
4 suppliers
inquiry