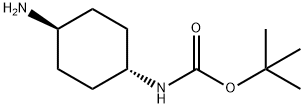
TRANS-N-BOC-1,4-CYCLOHEXANEDIAMINE synthesis
- Product Name:TRANS-N-BOC-1,4-CYCLOHEXANEDIAMINE
- CAS Number:177906-48-8
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
24424-99-5
832 suppliers
$13.50/25G
2615-25-0
272 suppliers
$5.00/1g

177906-48-8
216 suppliers
$6.00/250mg
Yield:177906-48-8 86%
Reaction Conditions:
in methanol at 0 - 20; for 16 h;
Steps:
Synthesis of tert-butyl ((1r,4r)-4-aminocyclohexyl)carbamate (Compound 114-01)
To a stirred solution of (1r,4r)-cyclohexane-1,4-diamine (1) (2 g, 17.54 mmol, 3.6 eq) in MeOH (50 mL) at 0° C. was added (Boc)2O (1.1 mL, 4.91 mmol, 1.0 eq).
The reaction mixture was stirred at room temperature for 16 h.
After completion of reaction by TLC, volatiles were evaporated and the residue was diluted with water and extracted with ethyl acetate (2*100 mL).
The combined organic layer was washed with brine solution (50 mL), dried over sodium sulphate and concentrated to afford tert-butyl ((1r,4r)-4-aminocyclohexyl)carbamate (Compound 114-01) (900 mg, yield: 86%). TLC system: MeOH/DCM (5:95), Rf value: ?0.3 (Ninhydrin stain); 1H NMR (400 MHz, CDCl3) δ 4.35 (brs, 1H), 3.39 (brs, 1H), 2.67-2.62 (m, 1H).
References:
Stingray Therapeutics, Inc.;Vankayalapati, Hariprasad;Sharma, Sunil;Kaadige, Mohan Rao;Weston, Alexis;Thode, Trason US2020/39979, 2020, A1 Location in patent:Paragraph 0417-0418