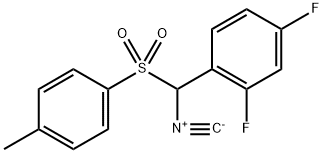
α-Tosyl-(2,4-difluorobenzyl)isocyanide synthesis
- Product Name:α-Tosyl-(2,4-difluorobenzyl)isocyanide
- CAS Number:660431-66-3
- Molecular formula:C15H11F2NO2S
- Molecular Weight:307.31
![Formamide, N-[(2,4-difluorophenyl)[(4-methylphenyl)sulfonyl]methyl]-](/CAS/20210305/GIF/668990-82-7.gif)
668990-82-7
1 suppliers
inquiry

660431-66-3
34 suppliers
$198.00/1g
Yield:660431-66-3 89.6 %
Reaction Conditions:
Stage #1: N-[(2,4-difluoro-phenyl)-(toluene-4-sulfonyl)-methyl]-formamidewith trichlorophosphate in tetrahydrofuran at 25;
Stage #2: with triethylamine in tetrahydrofuran at 20 - 30;Cooling with ice;
Steps:
1.2 2. Synthesis of Compound 3:
Add 80 g of Compound 2 into the reaction flask, then add 560 mL of anhydrous THF and 94 g of POCl3, and stir at 25° C. for 5 min. Slowly add 124g of triethylamine dropwise under ice-bath conditions, and after about 30-45min the dropwise addition is completed, and finally react at 20-30°C for 2h. TLC detection, post-treatment after disappearance of raw materials: drop 500mL water to quench, extract with ethyl acetate, wash the organic layer with saturated sodium bicarbonate solution and brine three times, dry over anhydrous sodium sulfate, filter and rotary evaporate under reduced pressure to remove the solvent to obtain yellow semi-solid. Then recrystallize with anhydrous n-propanol, obtain a yellow solid after suction filtration, and obtain 67.7 g of light yellow powder after drying, with a yield of 89.6% and a purity of 98.08%.
References:
CN115772110,2023,A Location in patent:Paragraph 0072-0073; 0076-0077
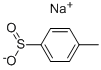
824-79-3
398 suppliers
$6.00/25g

660431-66-3
34 suppliers
$198.00/1g
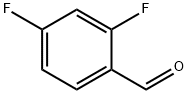
1550-35-2
377 suppliers
$6.00/5g

660431-66-3
34 suppliers
$198.00/1g
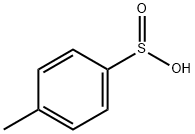
536-57-2
151 suppliers
$34.00/1g

1550-35-2
377 suppliers
$6.00/5g

660431-66-3
34 suppliers
$198.00/1g