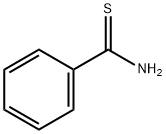
THIOBENZAMIDE synthesis
- Product Name:THIOBENZAMIDE
- CAS Number:2227-79-4
- Molecular formula:C7H7NS
- Molecular Weight:137.2

100-52-7
950 suppliers
$17.30/2g

2227-79-4
222 suppliers
$6.00/5g
Yield:2227-79-4 85%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium acetate;O,O-Diethyl hydrogen phosphorodithioate in 1,4-dioxane at 80 - 90; for 3 h;Inert atmosphere;
Steps:
General procedure for the one-pot synthesis of thioamides 3 and 4:
General procedure: to a solution of an aldehyde or a ketone (1.0 mmol) in dioxane (2 mL) was added hydroxylamine hydrochloride (1.0 mmol), sodium acetate (1.0 mmol), and O,O-diethyl dithiophosphoric acid (2) (1.0 mmol) under nitrogen atmosphere. The reaction mixture was stirred at 80-90 °C for 2-4 h, then cooled to rt, neutralized with aqueous sodium hydrogen carbonate and extracted with EtOAc (2 × 10 mL). The combined organic phase was dried over MgSO4, filtered, and evaporated under reduced pressure. The resulting crude product was purified by silica gel column chromatography using a gradient mixture of hexane/ethyl acetate as eluent to afford the corresponding thioamide (3 or 4). All the products are known compounds and were characterized by the comparison of their mp and spectral data with those reported in the literature. 10-12
References:
Yadav, Arvind K.;Srivastava, Vishnu P.;Yadav, Lal Dhar S. [Tetrahedron Letters,2012,vol. 53,# 52,p. 7113 - 7116]

55-21-0
389 suppliers
$5.00/5 g

2227-79-4
222 suppliers
$6.00/5g

100-47-0
362 suppliers
$14.14/25gm:

2227-79-4
222 suppliers
$6.00/5g
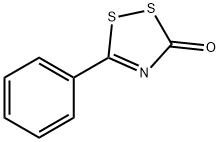
7047-10-1
4 suppliers
$92.79/1g
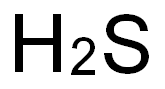
7783-06-4
73 suppliers
$133.00/25ml

2227-79-4
222 suppliers
$6.00/5g

110-91-8
687 suppliers
$9.00/1g
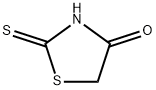
141-84-4
255 suppliers
$8.00/5g

55-21-0
389 suppliers
$5.00/5 g
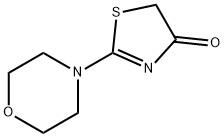
16781-67-2
6 suppliers
inquiry

2227-79-4
222 suppliers
$6.00/5g