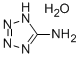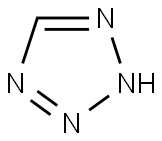
Tetrazole synthesis
- Product Name:Tetrazole
- CAS Number:288-94-8
- Molecular formula:CH2N4
- Molecular Weight:70.05
Tetrazole Synthesis: Nano-TiCl4.SiO2 (0.1 g) was added to a mixture of benzonitrile (1 mmol), sodium azide (2 mmol) in DMF (5 mL) at reflux for 2 h. After completion of reaction (as monitored by TLC), the mixture was allowed to cool to room temperature, the catalyst was removed by filtration. Then by adding ice water and 4N HCl (5 mL) to the residue, a white solid was obtained. This was then washed with cold chloroform. This simple procedure yielded pure tetrazole with good yields.

122-51-0
495 suppliers
$10.00/5ml

288-94-8
481 suppliers
$10.00/1ml
Yield:288-94-8 60.5%
Reaction Conditions:
with sodium azide;ammonium chloride;acetic acid at 95; for 18 h;
Steps:
1H-tetrazole (1)
NH4Cl (53.49 g, 1 mol, 1 eq.) and 97.51 g NaN3 (1.5 mol,1.5 eq.) were suspended in 258 cm3 triethyl orthoformate(1.55 mol, 1.55 eq.) and 500 cm3 acetic acid. The reactionmixture was stirred for 18 h at 95 C and filtrated aftercooling. After evaporation to dryness, the solid residue was extracted with 450 cm3 of boiling isopropanol. This stepwas repeated once. The off-white residue after evaporationof the combined isopropanol fractions was recrystallizedfrom ethanol. Yield: 42.4 g (60.5%). The spectroscopicproperties (1H NMR, 13C{1H} NMR, and MIR) werefound to be identical with the ones described in Ref
References:
Müller, Danny;Knoll, Christian;Weinberger, Peter [Monatshefte fur Chemie,2017,vol. 148,# 1,p. 131 - 137]
623-49-4
239 suppliers
$10.60/1gm:

288-94-8
481 suppliers
$10.00/1ml

74-90-8
4 suppliers
inquiry

288-94-8
481 suppliers
$10.00/1ml
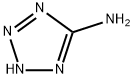
4418-61-5
459 suppliers
$10.00/5g

288-94-8
481 suppliers
$10.00/1ml