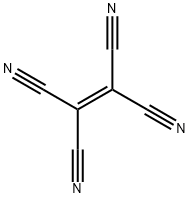
Tetracyanoethylene synthesis
- Product Name:Tetracyanoethylene
- CAS Number:670-54-2
- Molecular formula:C6N4
- Molecular Weight:128.09

109-77-3
428 suppliers
$10.00/5g

670-54-2
123 suppliers
$11.19/1G
Yield:670-54-2 37%
Reaction Conditions:
with selenium(IV) oxide in toluene at 100; for 1.25 h;
Steps:
Tetracyanoethylene (1).
Malononitrile, 6.61 g(0.1 mol), and selenium (IV) oxide, 16.65 g (0.15 mol),were added in succession to 75 mL of freshly distilled anhydrous toluene. The mixture was heated with stirring to 100°C (glycerol bath) and was kept for 75 min at that temperature. The mixture gradually turned yellow and then brown and dark red. In some cases, a small amount of yellow smoke was observed. When the reaction was complete, the mixture was heated to 110°C and filtered while hot through a filter paper, the filtrate was cooled, and the solvent was distilled off. The residue was 3.46 g (54%) of a red-brown material which was sublimed twice under reduced pressure at 130-140°C. Yield 2.37 g (37%),white crystals, mp 200-202°C; published data [12]:mp 199-200°C. IR spectrum, ν, cm-1: 2262, 2228,2220 (CN). 13C NMR spectrum (acetone-d6), δC, ppm:107.8 (CN), 111.9 (C=C).
References:
Kachanov;Slabko, O. Yu.;Kaminskii [Russian Journal of Organic Chemistry,2017,vol. 53,# 3,p. 462 - 464][Zh. Org. Khim.,2017,vol. 53,# 3,p. 452 - 453,2]
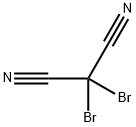
1885-23-0
5 suppliers
$144.00/100mg

670-54-2
123 suppliers
$11.19/1G
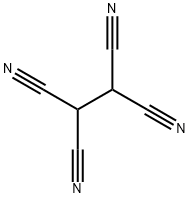
14778-29-1
4 suppliers
inquiry

670-54-2
123 suppliers
$11.19/1G
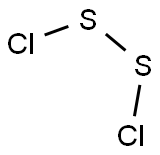
10025-67-9
95 suppliers
$40.60/50g

1885-23-0
5 suppliers
$144.00/100mg

670-54-2
123 suppliers
$11.19/1G
![Tetracyclo[6.6.2.02,7.09,14]hexadecane-2(7),3,5,9(14),10,12-hexene-15,15,16,16-tetracarbonitrile](/CAS/GIF/1625-84-9.gif)
