
Sorafenib synthesis
- Product Name:Sorafenib
- CAS Number:284461-73-0
- Molecular formula:C21H16ClF3N4O3
- Molecular Weight:464.82
4-Aminophenol anion was generated under a basic condition and compound 129 was added to the anion solution to give corresponding addition compound 131 in 87% yield. For an unknown reason, potassium carbonate used in the reaction increased the reaction rate significantly. Finally, compound 131 was condensed with isocyanate 132 in methylene chloride to give sorafenib (XVIII) in 92% yield as a white solid.

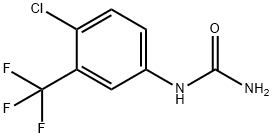
343247-69-8
47 suppliers
inquiry
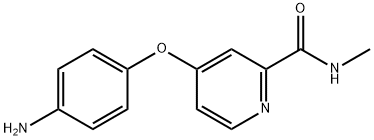
1154243-75-0
1 suppliers
inquiry

284461-73-0
531 suppliers
$11.00/1g
Yield:284461-73-0 97.9%
Reaction Conditions:
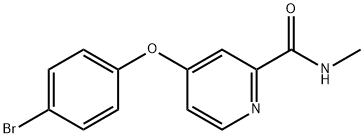
Stage #1: [4-(4-bromophenoxy)(2-pyridyl)]-N-methylcarboxamidewith potassium phosphate;tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 20; for 0.5 h;
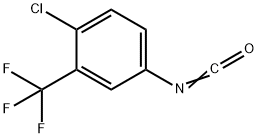
Stage #2: 4-chloro-3-trifluoromethylphenylurea in 1,4-dioxane at 80 - 100; for 1 h;Solvent;Reagent/catalyst;
Steps:
7-15 Preparation of Sorafenib (I)
Compound VI 28.72g, Pd2 (dba) 3 (0.0023 mol), 4,5-bisdiphenylphosphino-9,9-dimethylxanthene (0·0046 mol), 19.85g K3PO4, 1,4-dioxane 900ml, were added in a reaction vessel, stirred at room temperature for 30min, further, compound III 22.31 g was added, and the temperature was controlled at 80-100 ° C for 1 h, the reaction was monitored by TLC and the temperature was lowered to room temperature after the reaction was completed, the mixture was extracted with ethyl acetate, the purified water was washed, dried over anhydrous sodium sulfate and dried in vacuo obtain 42.60 g of sorafenib (I), yield 97.9%, purity 99.88%
References:
CN108341770,2018,A Location in patent:Paragraph 0015; 0046-0048; 0050
284462-37-9
389 suppliers
$8.00/1g
327-78-6
408 suppliers
$5.00/1g

284461-73-0
531 suppliers
$11.00/1g
![N-[4-Chloro-3-(trifluoromethyl)phenyl]-N'-(4-hydroxyphenyl)urea](/CAS/20180808/GIF/1129683-83-5.gif)
1129683-83-5
33 suppliers
inquiry
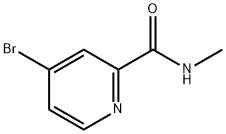
1209459-88-0
32 suppliers
$92.00/100mg

284461-73-0
531 suppliers
$11.00/1g

284462-37-9
389 suppliers
$8.00/1g
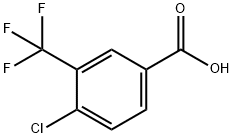
1737-36-6
138 suppliers
$15.00/1g

284461-73-0
531 suppliers
$11.00/1g
![4-[[2-[(methylamino)carbonyl]-4-pyridinyl]oxy]Benzoic acid](/CAS/20180703/GIF/827025-43-4.gif)
827025-43-4
3 suppliers
inquiry
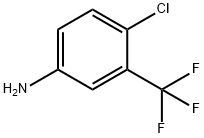
320-51-4
467 suppliers
$6.00/10g

284461-73-0
531 suppliers
$11.00/1g