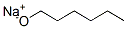
Sodium n-hexylate, in n-hexanol synthesis
- Product Name:Sodium n-hexylate, in n-hexanol
- CAS Number:19779-06-7
- Molecular formula:C6H13O*Na
- Molecular Weight:125.17
75-21-8
229 suppliers
$39.10/1mL
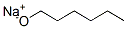
19779-06-7
6 suppliers
inquiry

111-27-3
509 suppliers
$5.00/25g

112-25-4
184 suppliers
$11.00/25g
Yield:-
Reaction Conditions:
in dodecane at 100; under 10501.1 Torr;Flow reactor;
Steps:
EXPERIMENTAL
General procedure: Our experiments were performed using commerciallyavailable C1-C7 and C10 alcohols, in combinationwith monoethylene glycol butyl ether (butyl cellosolve).The alcohols were dried according to standardprocedures using calcined zeolites, sodium sulfate,and calcium oxide. Dry ethanol was also used. Thecontent of water in the alcohols did not exceed0.2 wt %. The basic level of the product in the alcoholswas at least 99 wt %. The catalyst was sodium alkoxideof the corresponding alcohol. The kinetics of oxyethylation was studied in flowmixing and displacement reactors. The displacementreactor was described in [1]. The vast majority ofexperiments were performed in the displacement reactor.Our studies were performed at a pressure of1.4 MPa in the 60-180°C range of temperatures or(more often) at 80-150°C. The mixing reactor was ahollow cylindrical apparatus with a volume of 15 cm3.It was made of titanium and equipped with a magneticstirrer, an electrical heater, a thermocouple pocket,and tubes for input (at the bottom) and output (at thetop) of the reaction mixture. The reaction mixture wascooled to room temperature at the outlet of the reactor,using a water-jacket condenser. It then entered acollector from which samples could be taken. Theoperation to bring the reactor to a steady state was controlledby analyzing samples that started to be takenafter the quantity of the reaction mixture that hadpassed through the reactor reached at least six reactorvolumes. Preliminary calculations and our experimentsshowed that the pressure we used was sufficientto almost completely prevent the transition of EOfrom the liquid to vapor phase, the volume of which inthe device was reduced to a minimum. The materialbalance with respect to EO was reduced in experimentswith a maximum error of ±5%. In most cases,the total error in determining k0,obs and n did notexceed ±10 rel %. The specified temperature of thereactor was maintained to an accuracy of ±0.5 K. The initial mixtures and products of the reactionwere analyzed via gas-liquid chromatography. Sincethe reaction in all experiments was conducted in agreat excess of alcohol, the change in volume duringthe reaction was ignored. The molar concentrations ofthe reaction mixture’s components were calculatedusing the coefficients of volume expansion for eachcomponent.The experimental procedure was similar to the onedescribed in [2-4].
References:
Stul’, B. Ya. [Russian Journal of Physical Chemistry,2022,vol. 96,# 7,p. 1445 - 1456][Zh. Fiz. Khim.,2022,vol. 96,# 7,p. 1010 - 1022]
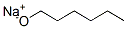
19779-06-7
6 suppliers
inquiry

1611-56-9
241 suppliers
$8.00/5g