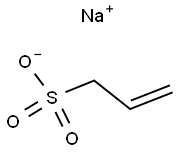
Sodium allylsulfonate synthesis
- Product Name:Sodium allylsulfonate
- CAS Number:2495-39-8
- Molecular formula:C3H5NaO3S
- Molecular Weight:144.12

98-29-3
537 suppliers
$7.00/25g

2495-39-8
357 suppliers
$6.00/25g
Yield:2495-39-8 98.93%
Reaction Conditions:
with sodium metabisulfite;N-benzyl-N,N,N-triethylammonium chloride;sodium hydroxide in water at 40 - 50; under 2250.23 Torr;Temperature;Reagent/catalyst;Pressure;
Steps:
2
930 g of 97% food grade sodium metabisulfite dissolved in 2500 g of deionized water, the temperature of the mixtureRaise to 50 ° C, stir until sodium metabisulfite is completely dissolved,Add 0.3g of triethylbenzyl chloride to the sodium metabisulfite solutionAmmonium and 0.3 g of polymerization inhibitor p-tert-butyl catechol,After stirring and mixing uniformly, a sodium metabisulfite mixture is obtained;The sodium metabisulfite mixture was passed through three high-precision plungers at a rate of 100 g/min, 1250 g of a 30% sodium hydroxide solution at a rate of 36.44 g/min, and 730 g of allyl chloride at a rate of 21.28 g/min. The metering pump is simultaneously pumped into the microchannel reactor for reaction.Adjust the reaction temperature in the microchannel reactor to 40 ° C,Adjusting the back pressure valve to make the pressure of the microchannel reactor0.3 MPa, the reaction-reacted crude product was filtered through a fine filter, and 538 g of a clear liquid was collected.The sample was analyzed by HPLC analysis, and the content of sodium allylsulfonate in the product obtained by the method of the present example was25.03%, the yield is 98.93% based on chloropropene.The Hull cell test piece passed the test.
References:
CN109232329,2019,A Location in patent:Paragraph 0023-0034
106-95-6
427 suppliers
$10.00/5g

2495-39-8
357 suppliers
$6.00/25g
107-05-1
411 suppliers
$16.80/100ML

2495-39-8
357 suppliers
$6.00/25g