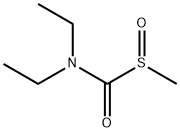
S-Methyl-N,N-diethylthiocarbamate Sulfoxide synthesis
- Product Name:S-Methyl-N,N-diethylthiocarbamate Sulfoxide
- CAS Number:140703-15-7
- Molecular formula:C6H13NO2S
- Molecular Weight:163.24
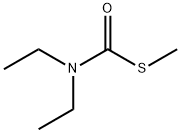
37174-63-3
20 suppliers
$155.00/10mg

140703-15-7
16 suppliers
$750.20/5MG
Yield:140703-15-7 80%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at -15 - -10;
Steps:
1-2 Example 1-2: /V,/V-diethyl-l-(methylsulfinyl)methanamide (1-2)
To a solution of A,A-diethyl-l-(methylsulfide)methanamide (26.7 g; 181.6 mmol) in 1L of dichloromethane cooled to -15 °C was added dropwise a solution of 77% m-chloroperbenzoic acid (36.6 g; 163.4 mmol) at a rate keeping the temperature of the reaction mixture not to exceed -10 °C during addition. Upon completion of addition, the reaction was cooled back to -15 °C, and the m- chlorobenzoic acid precipitate was removed by filtration. The filtrate was evaporated to dryness, and the crude product was subjected to chromatography (silica gel; methyl-t-butyl ether) followed by a second chromatography (silica gel; 10% MeOH in MTBE) afforded /V,/V-diethyl-l- (methylsulfinyl)methanamide Example 1-2 as a light yellow oil (23.8 g; 80%). 'H NMR (400 MHz, CDCb): d 3.63-3.53 (m, 2H, CH2), 3.51-3.42 (m, 2H, CH2), 2.76 (s, 3H, SCH3), 1.28 (t, 3H, J = 7.00 Hz, CH3), 1.17 (t, 3H, J = 7.20 Hz, CH3); 13C NMR (100 MHz, CDCb): d 168.0 (CO), 42.8 (CH2), 41.0 (CH2), 37.4 (SCH3), 14.5 (CH3), 12.5 (CH3); HPLC-MS: m/e 186.4 (M+Na+)
References:
WO2022/115742,2022,A1 Location in patent:Page/Page column 124-125
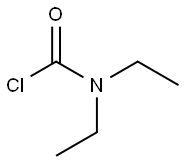
88-10-8
231 suppliers
$10.00/5g

140703-15-7
16 suppliers
$750.20/5MG

37174-63-3
20 suppliers
$155.00/10mg

140703-15-7
16 suppliers
$750.20/5MG
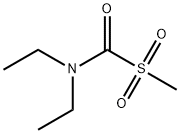
155514-79-7
16 suppliers
$140.00/5mg
