Ropivacaine synthesis
- Product Name:Ropivacaine
- CAS Number:84057-95-4
- Molecular formula:C17H26N2O
- Molecular Weight:274.4

106-94-5
564 suppliers
$10.00/5ml
27262-40-4
314 suppliers
$11.00/5g

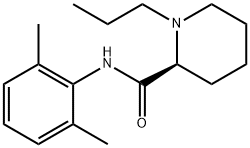
84057-95-4
216 suppliers
$49.00/10mg
Yield:84057-95-4 94%
Reaction Conditions:
in tetrahydrofuran; for 20 - 24 h;Heating / reflux;
Steps:
1
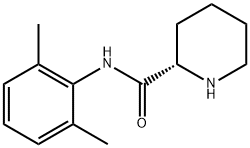
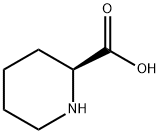
Example 1. Preparation of Ropivacaine baseRopivacaine is prepared starting from the intermediate (S) pipecolic acid 2,6-xylidide (145 g; 0.624 mols) and n-propyl bromide (766 g; 6.24 mols) in tetrahydrofuran (2.5 L) under reflux for approx. 20-24 hours. Inorganic salts are filtered off and the solvent is evaporated to dryness to obtain a dry- solid, consisting of crude Ropivacaine base. The solid is taken up into the minimum amount of diisopropyl ether (200 ml) and filtered under vacuum. The residue is washed on the filter with the same solvent (3 x 150 ml) and dried at 55°C under vacuum to obtain 161 g of Ropivacaine base in a 94% molar yield on the xylidide; HPLC purity = 99.75%; HPLC enantiomeric purity = 99.54%, concentration = 99.5%; loss on drying: 0.3%. EPO
References:
WO2006/133837,2006,A2 Location in patent:Page/Page column 3-4

98717-15-8
232 suppliers
$49.00/25mg

84057-95-4
216 suppliers
$49.00/10mg
33420-15-4
2 suppliers
inquiry

27262-40-4
314 suppliers
$11.00/5g

84057-95-4
216 suppliers
$49.00/10mg
3105-95-1
374 suppliers
$5.00/100mg

84057-95-4
216 suppliers
$49.00/10mg

87-62-7
454 suppliers
$10.00/5G

84057-95-4
216 suppliers
$49.00/10mg