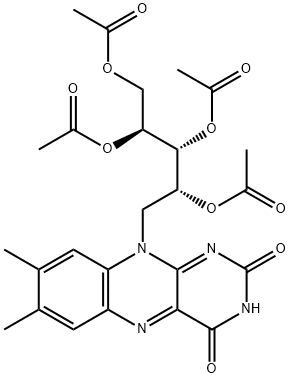
Riboflavin, 2',3',4',5'-tetraacetate synthesis
- Product Name:Riboflavin, 2',3',4',5'-tetraacetate
- CAS Number:752-13-6
- Molecular formula:C25H28N4O10
- Molecular Weight:544.51
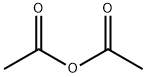
108-24-7
5 suppliers
$14.00/250ML
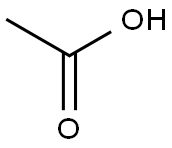
64-19-7
1563 suppliers
$10.00/25ML

83-88-5
788 suppliers
$5.00/1g

752-13-6
6 suppliers
inquiry
Yield: 80%
Reaction Conditions:
with perchloric acid at 45; for 0.666667 h;Schlenk technique;
Steps:
Synthesis of 2′,3′,4′,5′-Tetraacetylriboflavin (Rbf-I)
In a two-necked 50 mL Schlenk flask, riboflavin (0.5 g,1.33 mmol) was added to a 40 mL of solution containing a mixture of glacial acetic acid and acetic anhydride in (v:v, 1:1) volume percent. After the dropwise addition of 100 μL of 70% perchloric acid, the mixture was stirred for 40 min at 45 °C. The mixture was cooled in an ice bath and diluted with an equal volume of water and, then solution was extracted with chloroform (3 × 25 mL). The combined organic extracts were washed with deionized water (3 × 25 mL), followed with a saturated solution of NaCl. The combined organic phases was evaporated to dryness and, then the product was recrystallized from 95% ethanol, yielding 80%. FT-IR (KBr pellet, cm-1): 3158, 3032 (aromatic νC-H),2812 (aliphatic νC-H), 1749 (ester νC=O), 1663 (amide I.band νC=O), 1577 (aromatic νC=C), 1537 (amide II. bandνC=O), 1506, 1438, 1372, 1242, 1212, 1056, 839, 603 cm-1. 1H NMR (400 MHz, CDCl3 δ 7.27 ppm): δ = 8.89 (1H, s),8.00 (1H, s), 7.56 (1H, s), 5.66 (1H, d, J = 9.0 Hz), 5.47-5.38(3H, m), 4.89 (1H, s), 4.42 (1H, dd, J1 = 9.0 J2 = 2.9 Hz),4.26-4.21 (1H, m), 2.55 (3H, s), 2.43 (3H, s), 2.27 (3H, s),2.20 (3H, s), 2.06 (3H, s), 1.74 (3H, s) ppm. 13C NMR [100 MHz, CDCl3 δ 77.4 (3 peaks)]: δ = 171.5, 171.2,170.7, 170.6, 160.2, 155.4, 151.5, 137.6, 136.7, 135.2,133.6, 116.2, 70.8, 69.7, 69.3, 62.1, 45.1, 21.5, 21.1, 20.8,20.7, 20.3, 19.4 ppm.
References:
Saltan, G?zde Murat;K?ymaz, Deniz Aykut;Zafer, Ceylan;Din?alp, Haluk [Journal of Fluorescence,2017,vol. 27,# 6,p. 1975 - 1984]