
Raltegravir synthesis
- Product Name:Raltegravir
- CAS Number:518048-05-0
- Molecular formula:C20H21FN6O5
- Molecular Weight:444.42
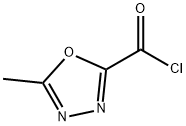
889131-28-6
14 suppliers
inquiry

518048-05-0
262 suppliers
$29.00/5mg
Yield:518048-05-0 95%
Reaction Conditions:
Stage #1:2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-6-oxo-4-pyrimidinecarboxamide with 4-methyl-morpholine in tetrahydrofuran at 0 - 5; for 0.166667 h;
Stage #2:5-methyl-1,3,4-oxadiazole-2-carboxylic acid chloride in tetrahydrofuran at 0 - 5; for 1.83333 h;
Steps:
1.8.B
To a 2 L round bottom flask free amine h (30 g) was added followed by THF (821 mL). The resulting slurry was cooled down to 0-50C, after which NMM (21.56 g) was added and the slurry so obtained was stirred for 10 minutes at the cold temperature. The previously prepared acyl chloride- containing slurry was added slowly to the free amine slurry over the course of 20 minutes such that the temperature did not exceed 5 0C. The slurry was then aged for 1.5 hours at 0-50C. At this time HPLC showed no more amine h (<0.5 % LCAP, 100% conversion). The reaction mixture was then quenched with KQH (17 % in water) (150 mL) which was added over the course of 10 minutes. The resulting yellow slurry was then stirred for an additional hour at temperatures less than 1O0C. The yellow slurry was then acidified to pH 3-4 with HCl (2N) (300 mL). To the resulting red wine colored solution, IPA (450 mL) was added. The low boiling point organic solvents were then evaporated under reduced pressure (40 torr) at room temperature to a final solution volume of 650 mL, at which volume crystalline Compound A began to precipitate. Water (350 mL) was then added to this new slurry over the course of 1 hour. The slurry was then cooled to 0-100C and aged for 2 hours. The aged slurry was filtered and the solid obtained was washed twice with water (100 mL each).. The solid so obtained was dried overnight under vacuum and nitrogen stream to give 38.3 g of Compound A (95% yield).
References:
MERCK & CO., INC. WO2007/87188, 2007, A2 Location in patent:Page/Page column 38-39

889131-28-6
14 suppliers
inquiry
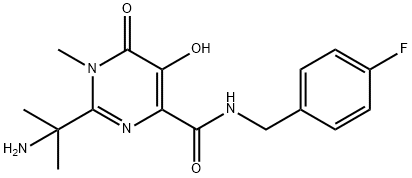
518048-03-8
151 suppliers
$17.00/250mg

518048-05-0
262 suppliers
$29.00/5mg
![4-Pyrimidinecarboxylic acid, 5-(benzoyloxy)-1,6-dihydro-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-, methyl ester](/CAS/20210111/GIF/1064707-18-1.gif)
1064707-18-1
0 suppliers
inquiry
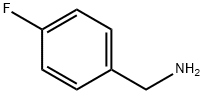
140-75-0
470 suppliers
$10.00/10g

518048-05-0
262 suppliers
$29.00/5mg
![Propanoic acid, 2,2-dimethyl-, 4-[[[(4-fluorophenyl)methyl]amino]carbonyl]-1,6-dihydro-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-5-pyrimidinyl ester](/CAS/20210305/GIF/1172131-66-6.gif)
1172131-66-6
1 suppliers
inquiry

518048-05-0
262 suppliers
$29.00/5mg

518048-03-8
151 suppliers
$17.00/250mg
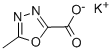
888504-28-7
226 suppliers
$10.00/250mg

518048-05-0
262 suppliers
$29.00/5mg