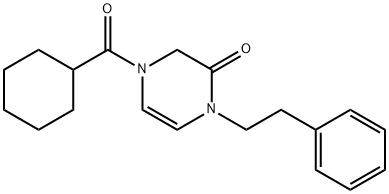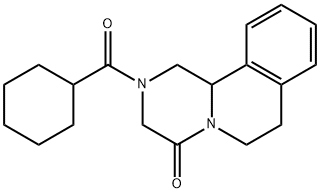
Praziquantel synthesis
- Product Name:Praziquantel
- CAS Number:55268-74-1
- Molecular formula:C19H24N2O2
- Molecular Weight:312.41

Another way of synthesizing this drug begins with isoquinoline, which is reacted with a mixture of cyclohexanecarbonyl chloride / potassium cyanide to make a dihydro derivative of isoquinoline (38.1.16). This is reduced by hydrogen over Raney nickel to give the reduction–reamidation product—the amide 1-(N-cyclohexylcarbonylaminomethyl)- 1,2,3,4-tetrahydroisoquinoline (38.1.17). Acylating this with chloracetic acid chloride gives a chlroacetyl derivative (38.1.18), which when heated in the presence of diethylamine results in an intramolecular alkylation, giving the desired product—prazi quantel.

Yield:55268-74-1 94.2%
Reaction Conditions:
with Sodium hydrogenocarbonate in ethyl acetate at 20 - 80; for 7 h;Temperature;
Steps:
1.S3; 4; 5.S3; 6.S3
S3, in the three-necked flask equipped with reflux condenser, thermometer and magnetic stirring device, add second step product 27.2g, ethyl acetate 300ml and sodium bicarbonate 20g, after stirring, slowly drip chloroacetyl chloride 12.4g, After the dropwise addition, the reaction was stirred at room temperature for 3 hours, then the temperature was raised to 80°C and the reaction was continued for 4 hours. After the reaction was completed, the reaction was cooled to room temperature, and the insolubles were removed by filtration. The solvent was distilled off, and the solid was collected to obtain the crude praziquantel. The crude product was recrystallized from acetone or ethanol to obtain 29.4 g of fine praziquantel, the yield was 94.2%.
References:
CN114195782,2022,A Location in patent:Paragraph 0010; 0033; 0036; 0043-0046; 0049-0050; 0053
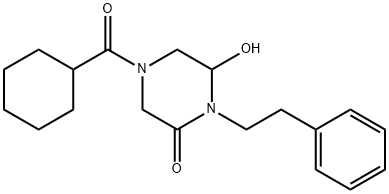
87693-75-2
2 suppliers
inquiry

55268-74-1
612 suppliers
$5.00/10mg

2719-27-9
351 suppliers
$16.00/25mL

55268-74-1
612 suppliers
$5.00/10mg
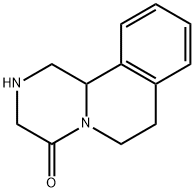
61196-37-0
66 suppliers
$281.79/250mg
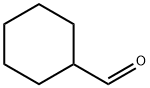
2043-61-0
336 suppliers
$6.00/5g

55268-74-1
612 suppliers
$5.00/10mg

![N-[(1,2,3,4-tetrahydro-1-isoquinolinyl)methyl]cyclohexanecarboxamide](/CAS/20180703/GIF/79848-93-4.gif)