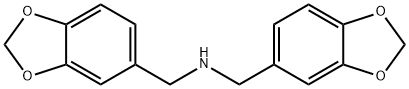
Piperonylamine synthesis
- Product Name:Piperonylamine
- CAS Number:2620-50-0
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
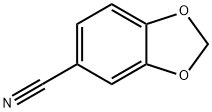
4421-09-4
153 suppliers
$8.00/1g

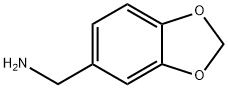
2620-50-0
164 suppliers
$20.00/10g
Yield:2620-50-0 100%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether for 2 h;Reflux;
Steps:
128 Example 128: 3-[(2H-l,3-benzodioxol-5-yl)methyl]-l-[(2,4- difluorophenyl)methyl] - 1 -( 1 -methylpiperidin-4-yl)urea (128)
2H-l,3-benzodioxole-5-carbonitrile (1.73 g, 11.75 mmol) in diethyl ether (15 ml) was added portion wise to a mixture of lithium aluminiumhydride (55.2 mmol hydride, 13.8 mmol, 525 mg) in diethyl ether (15 ml). The mixture was refluxed for 2 hours, then cooled and worked up (H20, 15 % NaOH, 3 x H20) and gave crude benzyl amine (1.77 g, quant.). This amine (1.0 g, 6.6 mmol) and pyridine (1.1 equiv., 17.6 mmol, 1.43 ml) was dissolved in dichloromethane (5.0 ml) and added dropwise to an ice cooled mixture of triphosgene (2.7 mmol, 801 mg,) in dichloromethane (5.0 ml). The mixture was stirred for 1 hour, then partitioned between cold 0.5 M sulfuric acid and dichloromethane. The organic phase was separated, dried and evaporated to give crude 5-(isocyanatomethyl)-2H-l,3-benzodioxole (1.08 g, 92 % yield). (0987) [00644] N-[(2,4-difluorophenyl)methyl]-l-methylpiperidin-4-amine (250 mg, 1.01 mmol) was dissolved in dichloromethane (1 ml) and 5-(isocyanatomethyl)-2H-l,3-benzodioxole (215 mg, 1.21 mmol) in dichloromethane (1 ml) was added in one portion. The mixture was stirred at room temperature for 1 hour and then purified by column chromatography using silicon dioxide gel, eluting with methanol to afford the title compound (275 mg, 66 % yield): NMR (400 MHz, Chloroform-;/) δ 7.21 (q, 1H), 6.87 - 6.73 (m, 2H), 6.73 - 6.59 (m, 3H), 5.92 (s, 2H), 4.57 (t, 1H), 4.40 (s, 2H), 4.29 (d, 2H), 4.26 (m, 1H), 2.88 (d, 2H), 2.27 (s, 3H), 2.07 (m, 2H), 1.75- 1.60 (m, 4H); LC-MS : 418.2 [M+H]+.
References:
ACADIA PHARMACEUTICALS INC.;BURSTEIN, Ethan, S.;OLSSON, Roger;BORGSTR?M, Bj?rn, Gustav;JANSSON, Karl, Erik;SK?LD, Niklas, Patrik;VON WACHENFELDT, Henrik;WAHLSTR?M, Larisa, Yudina WO2019/40107, 2019, A1 Location in patent:Paragraph 00642-00644
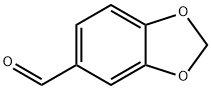
120-57-0
5 suppliers
$24.80/25g

2620-50-0
164 suppliers
$20.00/10g
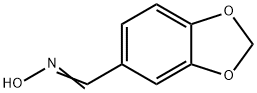
2089-36-3
46 suppliers
$47.70/5g

2620-50-0
164 suppliers
$20.00/10g
![5-(azidomethyl)benzo[d][1,3]dioxole](/CAS2/GIF/214783-17-2.gif)
214783-17-2
2 suppliers
inquiry

2620-50-0
164 suppliers
$20.00/10g