
Pifithrin-μ synthesis
- Product Name:Pifithrin-μ
- CAS Number:64984-31-2
- Molecular formula:C8H7NO2S
- Molecular Weight:181.21

536-74-3
307 suppliers
$10.00/1g

64984-31-2
119 suppliers
$25.00/10mg
Yield: 68%
Reaction Conditions:
Stage #1:phenylacetylene with lithium hexamethyldisilazane in tetrahydrofuran;acetone at -78; for 0.166667 h;Cooling with liquid nitrogen;
Stage #2: with N,N,N,N,N,N-hexamethylphosphoric triamide in tetrahydrofuran;acetone for 0.166667 h;
Stage #3: with sulphamoyl chloride in tetrahydrofuran;acetone at -78; for 1 h;
Steps:
3.2. Syntheses of Pifithrin-μ
Phenylacetylene (0.54 mL, 4.9 mmol) was added to anhydrous THF (15 mL) and cooled to 78 °C using a mixture of liquid nitrogen and acetone. Once cooled, a 1 M solution of LiHMDS (5.4 mL,5.4 mmol) was added dropwise and the mixture was left to stir for 10 min. HMPA (0.94 mL, 5.4 mmol)was then added before an additional 10 min of stirring. Freshly prepared sulfamoyl chloride (620 mg,5.4 mmol) dissolved in anhydrous THF (5 mL) was subsequently added and the reaction mixture wasleft to stir for 1 h, maintaining the temperature at 78 °C. After stirring and returning to RT, EtOAc(15 mL) was added and the mixture washed with aqueous NH4Cl (15 mL). The aqueous layer wasextracted with EtOAc (3 15 mL), the organic layers combined, dried over anhydrous Na2SO4 andconcentrated under reduced pressure. The resulting crude oil was subject to column chromatographyeluting with 30% EtOAc/hexane to yield pifithrin- as a white solid. Yield 0.603 g (68%). δH (400 MHz,DMSO-d6) 7.44 (3H, m, aromatic H), 7.36 (2H, d, 3J 8 Hz, aromatic H), 7.33 (2H, br s, NH2). δC (100 MHz,DMSO-d6) 146.1 (alkyne C), 144.9 (alkyne C), 138.4 (aromatic C), 129.2 (aromatic C), 127.6 (aromatic C),127.4 (aromatic C). (C8H7NO2S12 H2O requires C, 50.52; H, 4.24; N, 7.36%. Found: C, 50.86; H, 4.67;N, 6.99%); HPLC: C18 column, isocratic 60% acetonitrile/40% water as an eluent, retention time:8.63 min. Purity > 96%. MS (ESI-) m/z: 180.2.
References:
McKeon, Aoife M.;Egan, Alan;Chandanshive, Jay;McMahon, Helena;Griffith, Darren M. [Molecules,2016,vol. 21,# 7,art. no. 949]
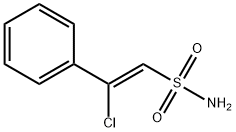
64984-30-1
14 suppliers
inquiry

64984-31-2
119 suppliers
$25.00/10mg
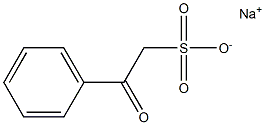
63738-92-1
21 suppliers
inquiry

64984-31-2
119 suppliers
$25.00/10mg
