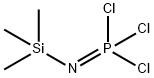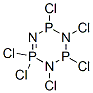
Phosphonitrilic chloride trimer synthesis
- Product Name:Phosphonitrilic chloride trimer
- CAS Number:940-71-6
- Molecular formula:Cl6N3P3
- Molecular Weight:347.66
874483-75-7
0 suppliers
inquiry
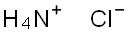
12125-02-9
1275 suppliers
$10.00/250G
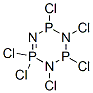
940-71-6
341 suppliers
$20.00/5G
Yield:940-71-6 71%
Reaction Conditions:
with pyridine;magnesium chloride in chlorobenzene at 80 - 132;Inert atmosphere;
Steps:
2 In a specific experiment, you can follow the following preparation process:
20.8 g (0.1 mol) of phosphorus pentachloride and 62.4 ml of anhydrous chlorobenzene were added to a 250 ml four-necked flask under nitrogen atmosphere, Slowly raised the temperature to 80 ° C, and stirred at 80 ° C for 1 to 2 hours until the phosphorus pentachloride was completely dissolved and stand-by. 5.88 g of ammonium chloride, 571 mg of magnesium chloride, 474 mg of pyridine and 62.4 ml of anhydrous chlorobenzene were placed in a 250 ml four-necked flask under nitrogen atmosphere, and slowly heated to a reflux state of 130 to 132 ° C under nitrogen atmosphere. The prepared phosphorus pentachloride chlorobenzene solution was slowly added dropwise to a 250 ml four-necked flask under reflux, and the dropping time was controlled to be not less than 4 hours. After the completion of the dropwise addition, stirring was continued under reflux for 1 to 2 hours; After cooling to room temperature, filtration, the mother liquid was concentrated to remove the solvent to obtain a crude compound hexachlorocyclotriphosphazene (referred to as "compound 2").The crude compound 2 obtained above and 10.5 ml of petroleum ether (boiling range 60 to 90) were added to a 50 ml jacketed bottle, Slowly raised the temperature to 80 ° C, stirred at 80 ° C for 1 to 2 hours until the crude product 2 was completely dissolved, and let stand to separate and remove a small amount of oil. The petroleum ether solution was then extracted twice with 40 ml of 98% concentrated sulfuric acid, and the concentrated sulfuric acid extract was diluted to 60% concentrated sulfuric acid solution with 22.7 ml of deionized water to precipitate a crude product of gray hexachlorocyclotriphosphazene. Crude compound 2 and 10.5 ml of petroleum ether (boiling range 60-90) were added to a 50 ml jacketed flask, slowly warmed to 80 ° C, and stirred at 80 ° C for 1 to 2 hours until the product was completely dissolved. Then, the temperature was slowly lowered to 0 to 5 ° C for crystallization, and after filtration, 8.22 g of Compound 2 as white crystals was obtained, yield 71%.The obtained compound was tested and found to be a cyclic hexachloro phosphazene compound having a specific crystal form.
References:
CN109134544,2019,A Location in patent:Paragraph 0221; 0224-0227
874483-75-7
0 suppliers
inquiry
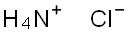
12125-02-9
1275 suppliers
$10.00/250G

7646-85-7
563 suppliers
$10.00/25ml
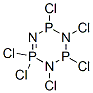
940-71-6
341 suppliers
$20.00/5G
874483-75-7
0 suppliers
inquiry
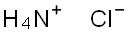
12125-02-9
1275 suppliers
$10.00/250G
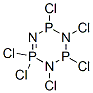
940-71-6
341 suppliers
$20.00/5G
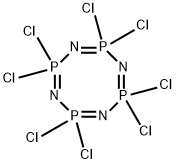
2950-45-0
15 suppliers
inquiry
874483-75-7
0 suppliers
inquiry
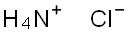
12125-02-9
1275 suppliers
$10.00/250G
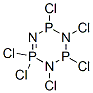
940-71-6
341 suppliers
$20.00/5G

2950-45-0
15 suppliers
inquiry
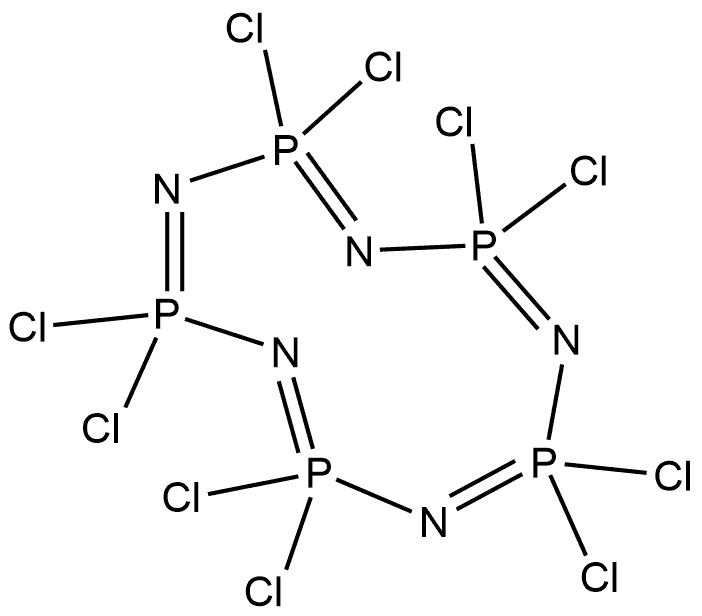
13596-41-3
0 suppliers
inquiry