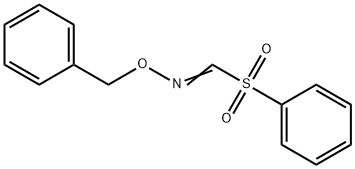
PHENYLSULFONYLMETHANAL O-BENZYL OXIME synthesis
- Product Name:PHENYLSULFONYLMETHANAL O-BENZYL OXIME
- CAS Number:177750-79-7
- Molecular formula:C14H13NO3S
- Molecular Weight:275.32
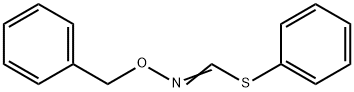
177750-77-5
0 suppliers
inquiry

177750-79-7
4 suppliers
inquiry
Yield:177750-79-7 95%
Reaction Conditions:
with sodium hydrogencarbonate;3-chloro-benzenecarboperoxoic acid in dichloromethane at 0 - 40; for 1.25 h;
Steps:
Preparation of (phenylsulfonyl)methanal O-benzyl oxime (6b)
Six equal portions of m-chloroperbenzoic acid (4.40 g, 25.5 mmol, 2.20 equiv) were added over 1 hto a suspension of phenyl N-(benzyloxy)methanimidothioate (2.82 g, 11.6 mmol, 1 equiv) and sodiumbicarbonate (2.00 g, 23.8 mmol, 2.05 equiv) in dichloromethane (50 mL) at 0 C. The resulting mixturewas stirred at 0 °C for 15 min. The reaction vessel was then placed in an oil bath that had been previouslyheated to 40 °C. The reaction mixture was stirred and heated at 40 °C for 1 h. The product mixture was allowed to cool down to 24 °C over 30 min and the cooled product mixture was transferred to a separatoryfunnel that had been charged with dichloromethane (100 mL) and a saturated aqueous sodium bicarbonatesolution (50 mL). The layers that formed was separated and the organic layer was washed with aqueoussaturated sodium thiosulfate solution (3 × 50 mL). The organic layer was dried and the dried solution wasfiltered. The filtrate was concentrated to dryness and the residue obtained was purified by flash-column chromatography (eluting with 10% ethyl acetate-hexanes initially, grading to 20% ethyl acetate-hexanes,linear gradient) to afford (phenylsulfonyl)methanal O-benzyl oxime (6b) as a white solid (3.03 g, 95%).
References:
Ma, Xiaoshen;Herzon, Seth B. [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2259 - 2265] Location in patent:supporting information
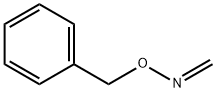
72399-18-9
1 suppliers
inquiry

177750-79-7
4 suppliers
inquiry
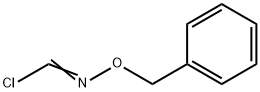
151451-59-1
0 suppliers
inquiry

177750-79-7
4 suppliers
inquiry