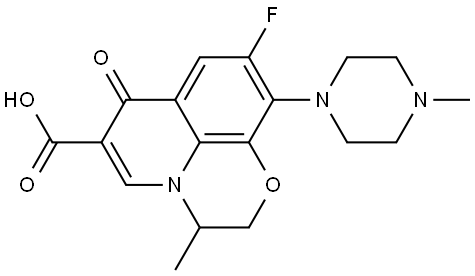
Ofloxacin synthesis
- Product Name:Ofloxacin
- CAS Number:82419-36-1
- Molecular formula:C18H20FN3O4
- Molecular Weight:361.37


109-01-3
659 suppliers
$5.00/5G
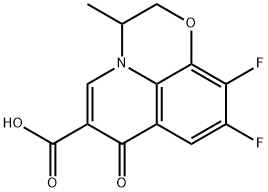
82419-35-0
257 suppliers
$10.00/250mg

82419-36-1
475 suppliers
$5.00/250mg
Yield:82419-36-1 95.8%
Reaction Conditions:
with potassium hydroxide in water at 60; for 6 h;Time;Solvent;
Steps:
9
3 g of starting material 9, 10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyridine[1,2,3,8] - [1,4] -benzoxazine-6-carboxylate, 4.58 water, 4.5 8 ^ methylpiperazine and 0.22 8 (81%)Potassium hydroxide, incubated at 60 ° C, and the reaction produced alcohol was removed during the reaction and the reaction was carried out for about 6 hours. The temperature is raised to the reflux reaction until the raw material disappears. N-methylpiperazine was completely substituted and N-methylpiperazine was recovered under reduced pressure. After acid, alkali pH adjustment, by extraction, washing, concentration and other steps. Finally, the concentrated solid was recrystallized from methanol, filtered and the mother liquor was concentrated and separated on a silica gel column (mobile phase methanol: dichloromethane = 1: 8). The solid was combined to give the ofloxacin product 3.44 g yeleld 95.8%. Compounds were determined by melting point, high molecular mass spectrometry, molecular weight, and nuclear magnetic resonance spectroscopy. The product was the same product as the product obtained in Example 7 and was anloxacin product.
References:
CN103755722,2016,B Location in patent:Paragraph 0062-0063

109-01-3
659 suppliers
$5.00/5G
![Ethyl 9,10-difluoro-3-methyl-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylate](/CAS/20180531/GIF/82419-34-9.gif)
82419-34-9
34 suppliers
$49.00/100mg

82419-36-1
475 suppliers
$5.00/250mg

109-01-3
659 suppliers
$5.00/5G
119-91-5
262 suppliers
$11.00/1g

6148-64-7
392 suppliers
$5.00/10g
94695-48-4
259 suppliers
$10.00/5G

82419-36-1
475 suppliers
$5.00/250mg
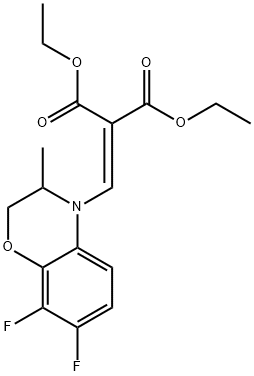
86760-99-8
0 suppliers
inquiry

82419-36-1
475 suppliers
$5.00/250mg
![Ethyl 9,10-difluoro-3-methyl-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylate](/CAS/20180531/GIF/82419-34-9.gif)
82419-34-9
34 suppliers
$49.00/100mg

82419-36-1
475 suppliers
$5.00/250mg