
OCTANENITRILE synthesis
- Product Name:OCTANENITRILE
- CAS Number:124-12-9
- Molecular formula:C8H15N
- Molecular Weight:125.21
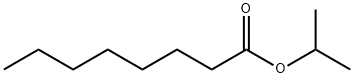
5458-59-3
60 suppliers
$36.00/25mL

124-12-9
84 suppliers
$21.00/5mL
Yield:124-12-9 82%
Reaction Conditions:
Stage #1:1-methylethyl octanoate with diisobutylaluminium hydride in hexane;dichloromethane at -78 - -60; for 2 h;Inert atmosphere;
Stage #2: with ammonia;iodine in tetrahydrofuran;hexane;dichloromethane;water at -60 - 20;Inert atmosphere;
Steps:
4.3. Typical procedure for the conversion of isopropyl esters (3) into nitriles (4)
General procedure: To a solution of isopropyl tetradecanoate (555 mg, 2.05 mmol) in CH2Cl2 (2 mL) was added DIBAL-H (1.04 M in hexane, 3 mL, 1.5 equiv) at -78 °C. The mixture was stirred for 3 h at -78 °C under argon atmosphere. Then, aq NH3 (6 mL), THF (3 mL), and I2 (1.85 mg, 7.30 mmol) were added. The resulting solution was warmed to room temperature and stirred for 3 h. Reaction mixture was poured into saturated aq Na2SO3 solution (10 mL) and extracted with chloroform (15 mL×3). The organic layer was dried over Na2SO4. After removal of the solvent under reduced pressure, the residue was purified by short column chromatography on silica gel to give tetradecanenitrile in 90% (386 mg).
References:
Suzuki, Yusuke;Yoshino, Takumi;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2011,vol. 67,# 21,p. 3809 - 3814] Location in patent:experimental part

929-55-5
1 suppliers
inquiry

124-12-9
84 suppliers
$21.00/5mL

124-13-0
360 suppliers
$7.00/25g

124-12-9
84 suppliers
$21.00/5mL
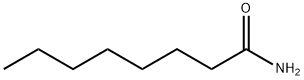
629-01-6
58 suppliers
$45.00/100mg

124-12-9
84 suppliers
$21.00/5mL

111-86-4
380 suppliers
$5.00/25g

124-12-9
84 suppliers
$21.00/5mL