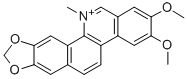
Nitidine chloride synthesis
- Product Name:Nitidine chloride
- CAS Number:13063-04-2
- Molecular formula:C21H18NO4+
- Molecular Weight:348.37

An isocyano group can serve as both a protecting group for the amino function, and, due to its electronic effect, as an activating group as well. These two functionalities are employed in a synthetic route whereby an amino function has to be protected and a condensation reaction is performed at the a-carbon atom, for which activation is required.
3,4-Fused tryptophan analogues 1563 and 1564 contain a ring that bridges the a-carbon and the 4-position of the indole ring, thus limiting the conformational flexibility of the side chain. The synthesis proceeds from N-formylated 40-bromotryptophan 1558 via isocyanide 1559, 2-propenoate 1560, and Pd-catalyzed cyclization of the a-2-propenyl dl-tryptophan derivatives 1561 and 1562 to give both the seven- and eight-membered constrained ring analogues 1564 and 1563. Dehydration of the formamide 1558 with triphosgene affords the isocyanide 1559 in 75% (87%) yield.
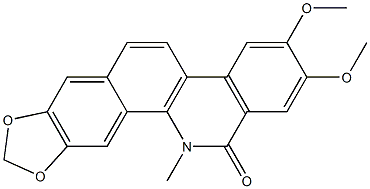
548-31-2
6 suppliers
inquiry

13063-04-2
200 suppliers
$39.00/5mg
Yield:13063-04-2 97%
Reaction Conditions:
Stage #1: 2,3-dimethoxy-12-methyl-[1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridin-13(12H)-onewith lithium aluminium hydride in tetrahydrofuran at 20; for 0.333333 h;Inert atmosphere;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate;
Steps:
1.5; 2 5) Synthesis of chlorinated nitidine
The intermediate 4 was dissolved in dry tetrahydrofuran (20 ml) under inert gas.Add lithium aluminum hydride (1~5 equivalents) and stir at room temperature for 20 minutes.The organic phase was collected by quenching with diethyl ether and concentrated.The residue is treated with 5 to 15% hydrochloric acid.The precipitated yellow solid precipitated is a chlorinated nitidine base.The yield was 97%.
References:
CN109942590,2019,A Location in patent:Paragraph 0016; 0020; 0021
![Formamide, N-[6-(3,4-dimethoxyphenyl)naphtho[2,3-d]-1,3-dioxol-5-yl]-N-methyl-](/CAS/20210305/GIF/105594-89-6.gif)
105594-89-6
0 suppliers
inquiry

13063-04-2
200 suppliers
$39.00/5mg
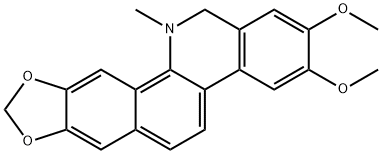
13063-06-4
3 suppliers
inquiry

13063-04-2
200 suppliers
$39.00/5mg
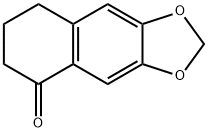
41303-45-1
9 suppliers
inquiry

13063-04-2
200 suppliers
$39.00/5mg
![[1,3]Benzodioxolo[5,6-c]phenanthridin-13(12H)-one, 2,3-dimethoxy-](/CAS/20210305/GIF/55171-31-8.gif)
