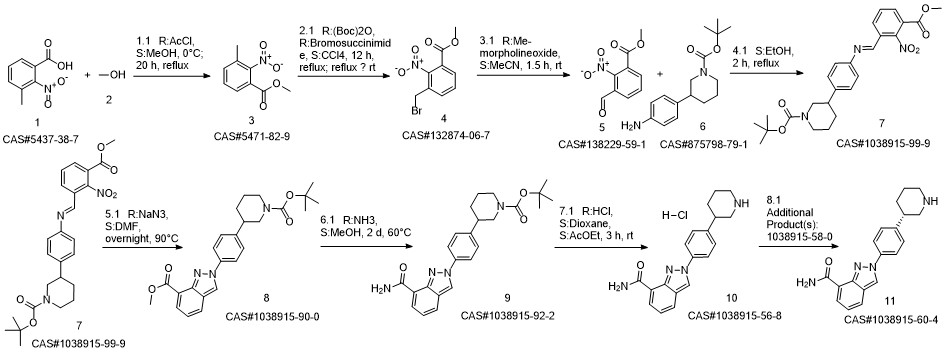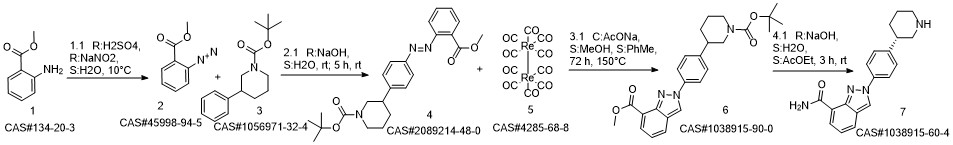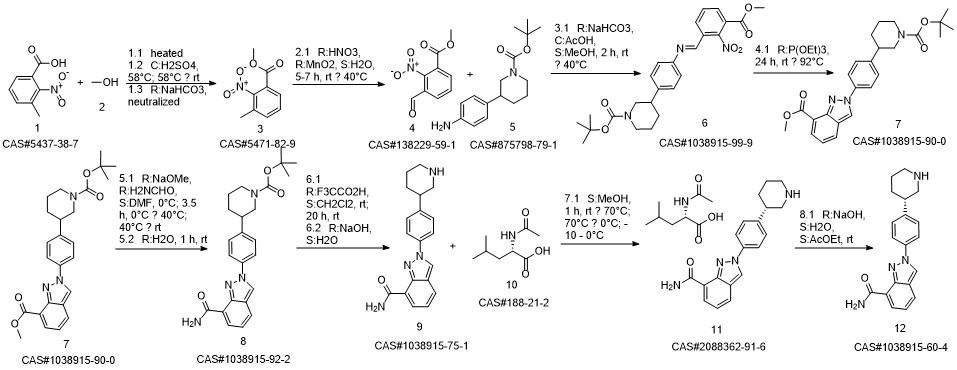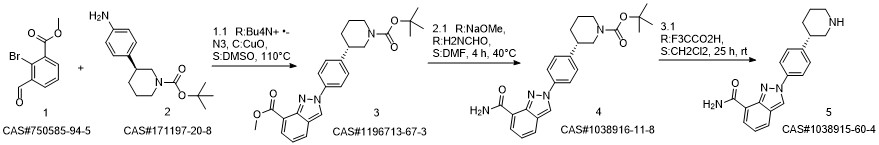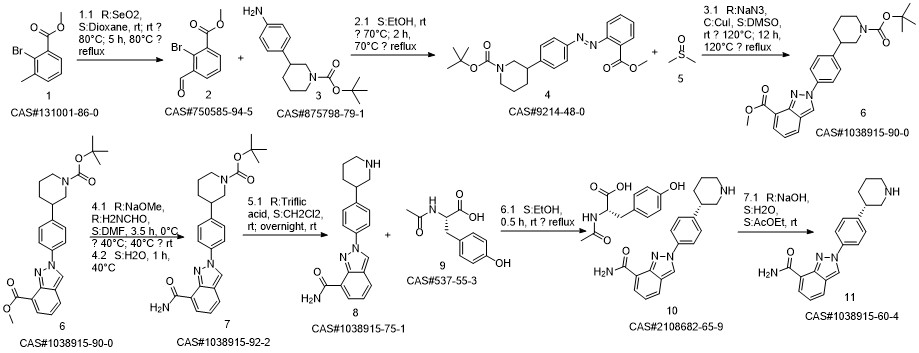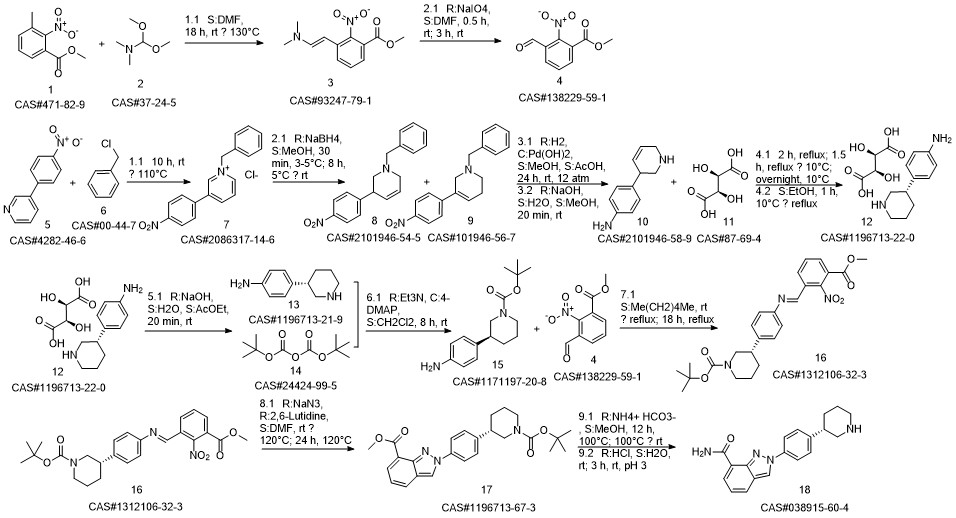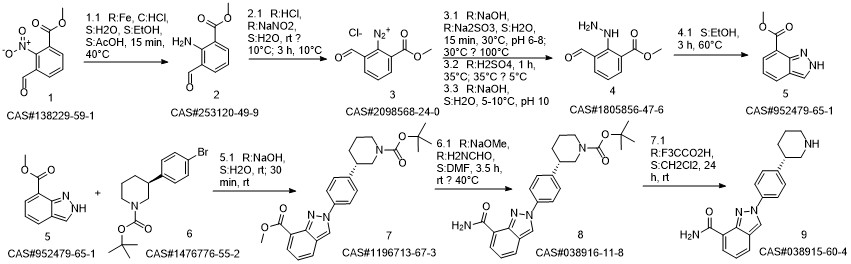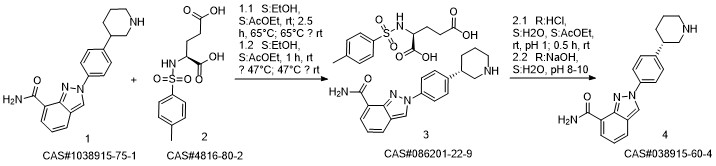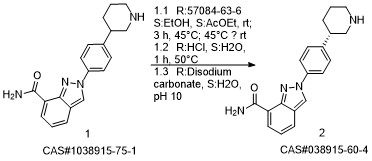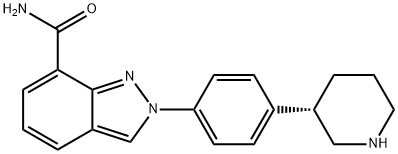
Niraparib synthesis
- Product Name:Niraparib
- CAS Number:1038915-60-4
- Molecular formula:C19H20N4O
- Molecular Weight:320.39
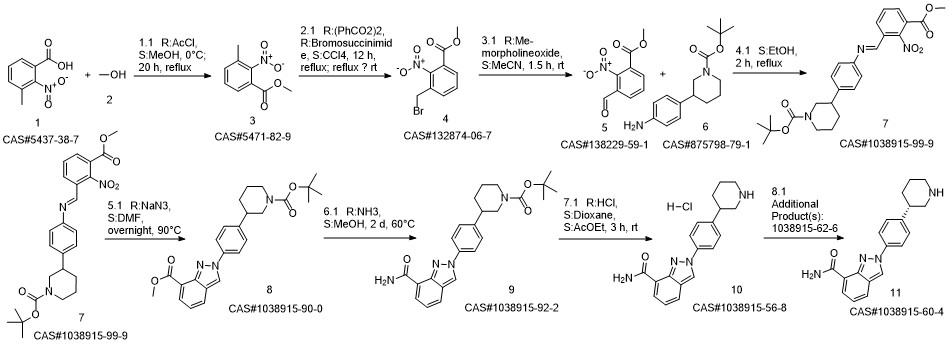
Reference: Jones, Philip; Ontoria Ontoria, Jesus Maria; Scarpelli, Rita; Schultz-Fademrecht, Carsten. Preparation of piperidinylphenylindazolylcarboxamide for use as poly(ADP-ribose)polymerase inhibitors. WO 2008084261. (Assignee Istituto Di Ricerche Di Biologia Molecolare P. Angeletti SpA, Italy)
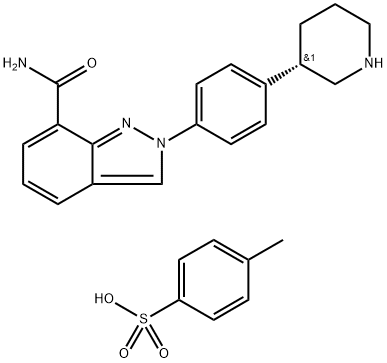
1038915-73-9
128 suppliers
$29.00/1mg

1038915-60-4
287 suppliers
inquiry
Yield:1038915-60-4 75.9%
Reaction Conditions:
with sodium hydroxide in 2-methyltetrahydrofuran at 20; for 0.5 h;
Steps:
1 Preparation of niraparib freebase (Form I)
To a mixture of 50.0 g (97.9 mmol) Niraparib tosylate monohydrate in 2-MeTHF (1L) was added 1% NaOH solution (500 mL) at room temperature. After the mixture was stirred for 30 minutes, the aqueous layer was separated and extracted with 2-MeTHF (0.5L, twice). The combined organic layer was washed with water (1L). The solution was concentrated under partial vacuum slowly below 30 °C until about 20 ml of suspension was left. The mixture was stirred for 30 minutes at room temperature and the solids were collected by filtration, to give 23.8 g (75.9%) of off-white crystalline solids (m.p. 189 °C). [M+H]+ at m/z 321, with the expected isotope pattern. Purity was found to be approximately 99.9% by HPLC. (0424) [000244] NMR (500.12 MHz, DMSO-de) d 9.27 (s, 1H), 8.59 (dd, 1H, J=l.8, 4.7 Hz), 8.07 (dd, 1H, J= 1.1, 7.0 Hz), 8.04 (d, 2H, J=8.7 Hz), 8.02 (dd, 1H, J=l.l, 8.1 Hz), 7.90 (br. s, 1H), 7.46 (d, 2H, J=8.5 Hz), 7.27 (dd, 1H, J=7.2, 8.3 Hz), 3.00 (br. d, 1H, J=l2.l Hz), 2.94 (br. d, 1H, J=l2.l Hz), 2.70 (m, 1H), 2.51 (m, 2H), 1.91, (d, 1H, J=l3.0 Hz), 1.68 (m, 1H), 1.61 (m, 1H), 1.49 (m, 1H). (0425) [000245] 13C NMR (125.77 MHz, DMSO-de) d 166.1, 146.5, 146.4, 138.0, 130.1, 128.7 (2C), 125.8, 123.9, 123.8, 122.3, 121.9, 121.1 (2C), 54.0, 46.4, 43.6, 32.3 and 27.0
References:
TESARO, INC.;STEWART, Alistair, James;WANG, Yi;WU, George;YIN, Jianguo WO2020/72796, 2020, A1 Location in patent:Paragraph 000242-000245
![methyl 2-{4-[(3S)-1-(tert-butoxycarbonyl)piperidin-3-yl]-phenyl}-2H-indazole-7-carboxylate](/CAS/20180703/GIF/1196713-67-3.gif)
1196713-67-3
5 suppliers
inquiry

1038915-60-4
287 suppliers
inquiry
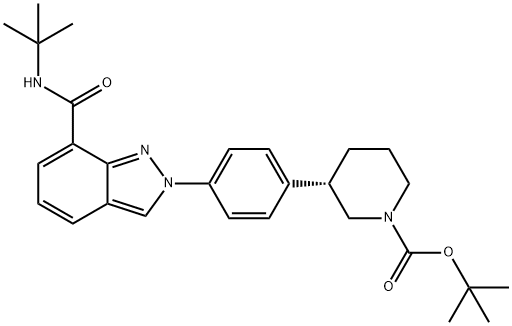
1476776-84-7
5 suppliers
inquiry

1038915-60-4
287 suppliers
inquiry
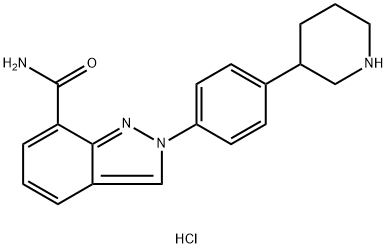
1038915-56-8
6 suppliers
inquiry

1038915-60-4
287 suppliers
inquiry
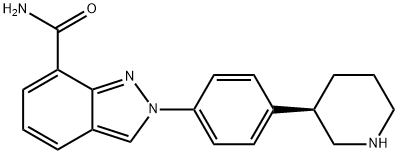
1038915-58-0
36 suppliers
inquiry

1038915-56-8
6 suppliers
inquiry

1038915-60-4
287 suppliers
inquiry