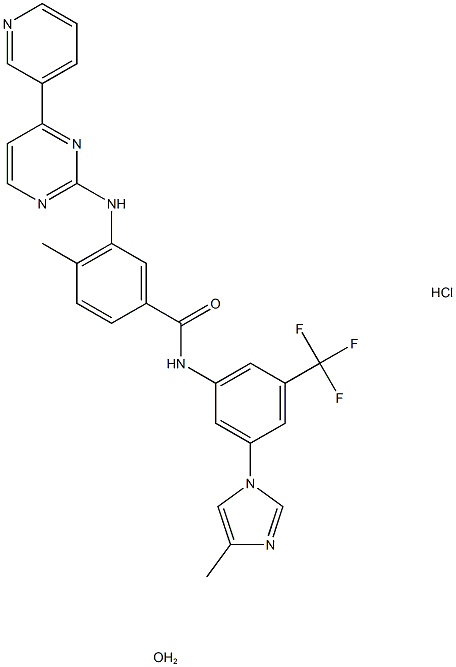
Nilotinib Hydrochloride Monohydrate synthesis
- Product Name: Nilotinib Hydrochloride Monohydrate
- CAS Number:923288-90-8
- Molecular formula:C28H25ClF3N7O2
- Molecular Weight:583.992
641571-10-0
421 suppliers
$21.00/5mg

923288-90-8
170 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;water in methanol at 30 - 50; for 0.75 h;Product distribution / selectivity;
Steps:
16
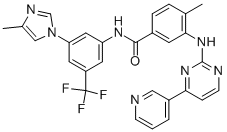
Example 16[0080] A l L, 4-neck, round-bottom flask equipped with a mechanical stirrer, a thermometer, heating/cooling capacity, and an addition funnel was charged in sequence with 4-methyl-N- [3 -(4-methyl-imidazol- 1 -yl)-5 -trifluoromethyl-phenyl] -3 -(4-pyridin-3 -yl-pyrimidin-2- ylamino)-benzamide free base (10 g), methanol (250 mL), and 37% hydrochloric acid (1.85 g) under nitrogen purge. The mixture was heated to 42-5O0C and stirred for an additional 15 minutes. The resulting solution was filtered through a polypropylene pad, while maintaining the batch temperature above 4O0C. The clear solution was transferred under nitrogen atmosphere to another 1 L, 4-neck, round-bottom flask equipped with a mechanical stirrer, a thermometer, and heating/cooling capacity. The batch was stirred and cooled to 3O0C over a period of 30 minutes. Seeds (20 mg) were added at this temperature, and the batch was cooled to 230C over a period of 45 minutes. The batch was stirred for an additional 3 hours to obtain a thick white suspension. The suspension was cooled to -1O0C over a period of 1.5 hours and stirred for an additional 30 minutes. Any solid was collected by filtration and rinsed with cold (-1O0C) methanol (20 mL). The solid was dried at 50-55°C/10-20 torr for 8-16 hours to obtain 4-methyl-N-[3v(4-methyl-imidazol-l-yl)-5-trifluoromethyl-phenyl]-3-(4- pyridin-3-yl-pyrirnidin-2-ylamino)-benzamide monohydrochloride monohydrate salt form B (9.8 g) as a white solid.[0081] 1H NMR 300 MHz, DMSO-d6), δ 10.9 (s, IH), 9.58 (s, IH), 9.29 (s, IH), 9.20 (s, IH), 8.70 (d, IH), 8.63 (s, IH), 8.55 (d, IH), 8.49 (d, IH), 8.32 (d, 2H), 8.00 (s, IH), 7.91 (s, IH), 7.84 (d, IH), 7.56-7.44 (m, 3H), 2.50 (s, 3H), 2.35 (s, 3H); x-ray diffraction pattern showing maxima at 2Θ = 7.4°, 9.4°, 11.6°, 12.1°, 15.8°, 19.3°, 19.6°, 22.1°, 24.1°, 25.7°.
References:
WO2007/15870,2007,A2 Location in patent:Page/Page column 28