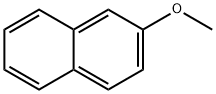
Naphthalene, 2-iodo-6-methoxy- (9CI) synthesis
- Product Name:Naphthalene, 2-iodo-6-methoxy- (9CI)
- CAS Number:67886-69-5
- Molecular formula:C11H9IO
- Molecular Weight:284.09

5111-65-9
347 suppliers
$6.00/5g

7732-18-5
472 suppliers
$13.50/100ML

67886-69-5
13 suppliers
inquiry
Yield:67886-69-5 805 mg (67%)
Reaction Conditions:
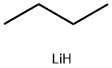
with iodine;tert.-butyl lithium in tetrahydrofuran;pentane;
Steps:
13.A A.
A. 6-Iodo-2-methoxynaphthalene To a solution of 1.00 g (4.22 mmol, Aldrich) of 6-bromo-2-methoxynaphthalene in 10 ml of dry THF at -78° was added dropwise 4.5 ml (1.4M in pentane, 6.3 mmol, Aldrich) of t-butyllithium solution over 10 minutes. The reaction mixture was stirred at -78° for 30 minutes then at 0° for 15 minutes. The resulting yellow solution was re-cooled to -78° then 1.20 g (4.72 mmol, Aldrich) of iodine was added in one portion. The reaction mixture was warmed to room temperature, stirred for 1 hour then added to 50 ml of H2 O and extracted with 50 ml of ethyl acetate. The organic extract was washed with an additional 50 ml of H2 O, dried (MgSO4) and concentrated in vacuo to give a solid. The crude solid was recrystallized (EtOAc/petroleum ether) to afford 805 mg (67%) of title compound as pale yellow flakes, m.p. 142°-143°. IR(KBr) 1622, 1578, 1494, 1264, 112, 1165, 1029, 898, 854, 817, 476, 465 cm-1.
References:
US4910208,1990,A
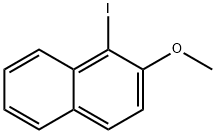
93-04-9
461 suppliers
$13.00/25g

67886-69-5
13 suppliers
inquiry

5111-65-9
347 suppliers
$6.00/5g

67886-69-5
13 suppliers
inquiry
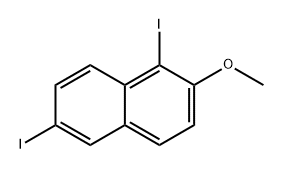
32721-21-4
33 suppliers
$99.00/500mg

93-04-9
461 suppliers
$13.00/25g

67886-69-5
13 suppliers
inquiry
97825-82-6
0 suppliers
inquiry