
N-Octyl pyrrolidone synthesis
- Product Name:N-Octyl pyrrolidone
- CAS Number:2687-94-7
- Molecular formula:C12H23NO
- Molecular Weight:197.32
N-Octylpyrrolidone (NOP) is a highly efficient and selective solvent that can be used in the synthesis of fine chemicals such as medicines, pesticides, pigments, fragrances and cleaning agents. Its synthesis method is to slowly add chloro-n-octane dropwise to the mixture of 2-pyrrolidone and tetrabutylammonium bromide at 85°C for 10 h, and the mixtrue was stirred for at 90°C to give N-octylpyrrolidone (NOP).
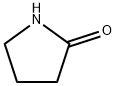
616-45-5
384 suppliers
$14.00/5g

111-83-1
500 suppliers
$12.00/25g

2687-94-7
346 suppliers
$17.34/25ML
Yield:-
Reaction Conditions:
with NaH in toluene;Petroleum ether
Steps:
1 EXAMPLE 1
EXAMPLE 1 Preparation of 1-n-Octylazacyclopentan-2-one having the following structure: STR5 A suspension of 5.44 g of 57% sodium hydride/mineral oil (actually 3.10 g NaH, 0.13 mole) in 200 ml of petroleum ether was allowed to settle and the residue washed with 2 * 200 ml of petroleum ether. After the excess petroleum ether was removed, 150 ml of dry toluene was added and the mixture stirred as 10 g (0.1174 mole) of azacyclopentan-2-one in 25 ml of dry toluene was added dropwise over one hour. After the addition was complete, the mixture was refluxed for one hour then 25.1 g (0.13 mole) of 1-bromooctane in 25 ml of dry toluene was added dropwise over one hour to the refluxing mixture. After the addition was complete, the mixtures was refluxed for 3 days. The reaction mixture was filtered twice (the latter time through celite), and the solvent evaporated to yield a yellow oil. Vacuum distillation yeilded a fraction b.p. 128°-132° at 0.3 mm, weighing 13.6 g (59%).
References:
Nelson Research & Development Company US4122170, 1978, A

616-45-5
384 suppliers
$14.00/5g

124-13-0
371 suppliers
$7.00/25g

2687-94-7
346 suppliers
$17.34/25ML

616-45-5
384 suppliers
$14.00/5g

111-85-3
392 suppliers
$5.00/25g

1634-04-4
666 suppliers
$14.00/100g

2687-94-7
346 suppliers
$17.34/25ML