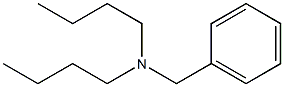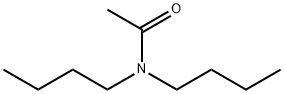
N,N-DI-N-BUTYLACETAMIDE synthesis
- Product Name:N,N-DI-N-BUTYLACETAMIDE
- CAS Number:1563-90-2
- Molecular formula:C10H21NO
- Molecular Weight:171.28

111-92-2
393 suppliers
$14.00/25mL

75-36-5
578 suppliers
$17.92/100G

1563-90-2
55 suppliers
$144.00/25mg
Yield: 76.5%
Reaction Conditions:
with triethylamine in chloroform for 2 h;Reflux;
Steps:
2.2 Ligand Synthesis
General procedure: DBAA and DBBA were synthesized through the reaction of acetyl chloride (98%, AlfaAesar) or butyryl chloride (99%, Acros Organics), respectively, in chloroform with equimolardibutylamine (99.5%, Sigma-Aldrich) in the presence of equimolar triethylaminebase (99%, Acros Organics) in a stirred ice bath. The solution was then heated to reflux at the boiling point of chloroform for at least 2 h. The organic product was washed withdeionized water, 10 wt% Na2CO3solution, 1.2 mol·L-1 HCl solution and a final deionizedwater wash. The organic layer was dried over anhydrous Na2SO4,filtered, and the solventwas removed under reduced pressure. Both ligands were > 95% pure. The DBAA yield was76.5%, and the DBBA yield was 81.9%. DBAA 1H NMR (500 MHz, CDCl3):δ 0.68 (m,6H), 1.07 (m, 4H), 1.27 (m, 4H), 1.82 (s, 3H), 2.98 (t, 2H), 3.05 (t, 2H).
References:
Canner, Adam J.;Harwood, Laurence M.;Cowell, Joseph;Babra, Jasraj S.;Brown, Solomon F.;Ogden, Mark D. [Journal of Solution Chemistry,2020,vol. 49,# 1,p. 52 - 67]
581-55-5
44 suppliers
$45.00/250mg

111-92-2
393 suppliers
$14.00/25mL

1563-90-2
55 suppliers
$144.00/25mg

102-82-9
8 suppliers
$10.00/1ml
108-24-7
5 suppliers
$14.00/250ML

1563-90-2
55 suppliers
$144.00/25mg
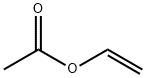
108-05-4
574 suppliers
$4.00/1000g

111-92-2
393 suppliers
$14.00/25mL

1563-90-2
55 suppliers
$144.00/25mg