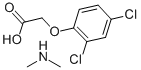
N-Methylmethanamine 2,4-dichlorophenoxyacetate synthesis
- Product Name:N-Methylmethanamine 2,4-dichlorophenoxyacetate
- CAS Number:2008-39-1
- Molecular formula:C10H13Cl2NO3
- Molecular Weight:266.12

94-75-7
484 suppliers
$10.00/5g

124-40-3
510 suppliers
$18.00/100ml

2008-39-1
107 suppliers
$355.00/10g
Yield:2008-39-1 100%
Reaction Conditions:
Stage #1:2,4-Dichlorophenoxyacetic acid with ethylenediaminetetraacetic acid in tert-butyl methyl ether
Stage #2:dimethyl amine in tert-butyl methyl ether; pH=8 at 56;Product distribution / selectivity;
Steps:
6
Example 6; Process[0046] MTBE was charged into a reactor equipped with the overhead reflux condenser and a stirrer. The phenoxyacid, REAX83 and EDTA were added. The mixture was stirred for 15-20 minutes. Anhydrous DMA was added till solution reaches and remained at pH of above 8. During introduction of DMA, the reaction temperature rose to the boiling point of the MTBE (56°C). Product precipitation occurred during introduction of DMA. The resulting product slurry was cooled by water to 25°C and the precipitate collected as a wet cake in a centrifuge. The wet cake from centrifuge was dried in a drier while the mother liquor was recycled to the reactor for next batch. Fresh MTBE was added to the reactor to compensate for MTBE losses.Conclusion[0047] Table 1 below provides comparison for the ether and acetone processes. Table 1CE-A Example 6 (Acetone Process) (Ether Process)2,4-D (tech.) charge (kg) 4,000 4,000(bags) 5 5Reaction mass (kg) 14022 12829S.G. of solvent, g/mL 0.791 0.739Mass of dray final product (Yield) (Kg) 4341 4875Isolated yield (%) 92.5 100Active as 2,4-D (%) 82.4 79.8Formulation in situ Not Possible Yes
References:
NUFARM AUSTRALIA LIMITED;KRAVETS, Eduard WO2011/143690, 2011, A1 Location in patent:Page/Page column 10-11
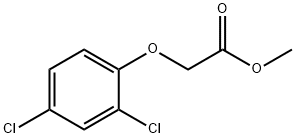
1928-38-7
85 suppliers
$60.00/2 g

124-40-3
510 suppliers
$18.00/100ml

2008-39-1
107 suppliers
$355.00/10g
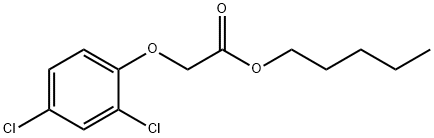
1917-92-6
2 suppliers
inquiry

124-40-3
510 suppliers
$18.00/100ml

2008-39-1
107 suppliers
$355.00/10g

94-75-7
484 suppliers
$10.00/5g

124-40-3
510 suppliers
$18.00/100ml
74-89-5
7 suppliers
$13.44/25ML

2008-39-1
107 suppliers
$355.00/10g
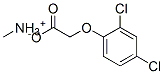
51173-63-8
0 suppliers
inquiry
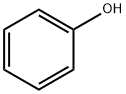
108-95-2
737 suppliers
$14.00/25g

2008-39-1
107 suppliers
$355.00/10g
