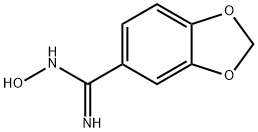
N-HYDROXY-1,3-BENZODIOXOLE-5-CARBOXIMIDAMIDE synthesis
- Product Name:N-HYDROXY-1,3-BENZODIOXOLE-5-CARBOXIMIDAMIDE
- CAS Number:4720-72-3
- Molecular formula:C8H8N2O3
- Molecular Weight:180.16
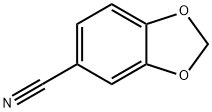
4421-09-4
153 suppliers
$8.00/1g

4720-72-3
41 suppliers
$45.00/100mg
Yield:4720-72-3 94%
Reaction Conditions:
with hydroxyamino hydrochloride;N-ethyl-N,N-diisopropylamine in ethanol at 20; for 21.5 h;
Steps:
1.6
DIPEA (12.17 ml, 69.7 mmol) was added to a suspension of piperonylonitrile (5 g, 34.0 mmol) and hydroxylamine hydrochloride (4.72 g, 68.0 mmol) in EtOH (75 mL). After stirring at 20° C. for 21.5 h, the reaction mixture was concentrated to dryness. The resulting colorless oil was cooled to 0° C. and cold water was added (75.0 mL) and the mixture was stirred for 30 minutes to give a white slurry. The solids were filtered, washed with water (2×15 mL) and the resulting solid dried at 40° C. under high vacuum until constant weight to afford Intermediate 6a (Z)-N′-hydroxybenzo[d][1,3]dioxole-5-carboximidamide (5.76 g, 94% yield) as a white solid: 1H NMR (DMSO-d6) is consistent with the desired product; MS m/z 181.1 (MH+); HPLC 100%, Rt=0.16 (0.31) min. Alternatively DIPEA can be replaced by sodium carbonate or commercial sodium ethoxide while working in ethanol as solvent for the reaction. Except when commercially available, all N′-hydroxycarboximidamides cited in the examples hereafter were prepared following the recipe outline before for intermediate 6a with the appropriate nitrile precursor and including its variation on the nature of the base used.
References:
US2015/133495,2015,A1 Location in patent:Paragraph 0164; 0165
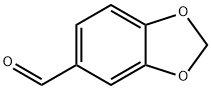
120-57-0
5 suppliers
$28.39/25g

4720-72-3
41 suppliers
$45.00/100mg
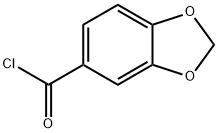
25054-53-9
92 suppliers
$27.57/250mgs:

4720-72-3
41 suppliers
$45.00/100mg
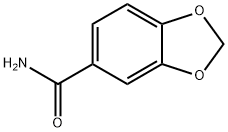
4847-94-3
42 suppliers
$28.60/25MG

4720-72-3
41 suppliers
$45.00/100mg
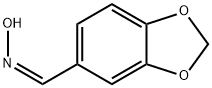
20747-42-6
0 suppliers
inquiry

4720-72-3
41 suppliers
$45.00/100mg