
N-Didesmethyl Loperamide synthesis
- Product Name:N-Didesmethyl Loperamide
- CAS Number:66164-06-5
- Molecular formula:C27H29ClN2O2
- Molecular Weight:448.98
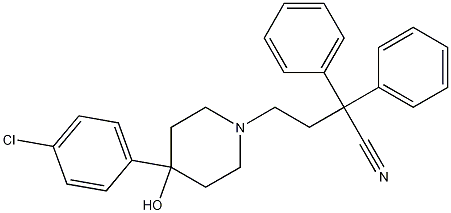
63959-33-1
7 suppliers
inquiry

66164-06-5
18 suppliers
$185.00/5mg
Yield:66164-06-5 87%
Reaction Conditions:
with potassium hydroxide in tert-butyl alcohol at 100; for 72 h;Inert atmosphere;
Steps:
4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-2,2-diphenylbutanamide (b)
4-(4-Chlorophenyl)-4hydroxypiperidin-1-yl)-2,2- diphenylbutanenitrile (1.5 g, 3.5 mmol) was dissolved in tbutanol (10 mL) and potassium hydroxide (589.15 mg, 10.5 mmol) was added. The reaction mixture was stirred at 100°C for 3 days. After concentration under vacuum, the crude material was re-dissolved in dichloromethane and filtered through a pad of celite. After evaporate the organic solvent, the residue was purified on silica gel column eluted with MeOH: CH2Cl2 (5:95 v/v) to give 1.37 mg (3.05 mmol, 87% yield) compound b as a white solid. TLC (silica gel; MeOH: CH2Cl2 (5:95 v/v)); Rf=0.2. 1H NMR (CDCl3): δ 7.39 (d, J=4.8Hz, 2H), 7.29 (m, 12H), 6.56 (s, 1H), 5.58 (s, 1H), 2.79 (d, J=11.37 Hz, 2H), 2.67(t, J=7.67 Hz, 2H), 2.44 (m, 4H), 2.09 (t, J=12.64 Hz, 2H), 1.70 (d, J=11.91 Hz, 2H). 13C NMR (CDCl3): δ 175.89, 145.92, 142.45, 132.03, 127.92, 127.65, 126.31, 125.37, 69.96, 59.11, 54.16, 48.70, 37.31, 34.91. LC- MS, [M+H]+, 449.3, Calculated for C27H30ClN2O2 [M++H], 449.1990.
References:
Bao, Xiaofeng;Liu, Duliang [Tetrahedron Letters,2013,vol. 54,# 11,p. 1412 - 1415] Location in patent:supporting information

39186-58-8
224 suppliers
$6.00/5g

66164-06-5
18 suppliers
$185.00/5mg

39512-49-7
312 suppliers
$29.69/1g

66164-06-5
18 suppliers
$185.00/5mg

39512-49-7
312 suppliers
$29.69/1g

39186-58-8
224 suppliers
$6.00/5g

66164-06-5
18 suppliers
$185.00/5mg