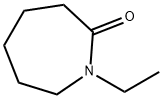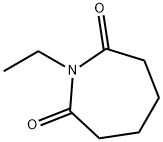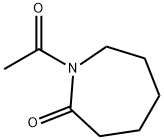
N-Acetylcaprolactam synthesis
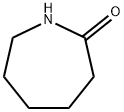
- Product Name:N-Acetylcaprolactam
- CAS Number:1888-91-1
- Molecular formula:C8H13NO2
- Molecular Weight:155.19
Yield:1888-91-1 94%
Reaction Conditions:
in toluene at 100; for 0.5 h;Temperature;
Steps:
1-4 Example 4
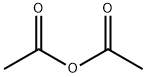
At room temperature, 1 mol of caprolactam (113.2 g) was dissolved in 1.25 mol of toluene (115.2 g) to prepare a solution.A solution was prepared by dissolving 1.2 mol of acetic anhydride (122.5 g) in 1.25 mol of toluene (115.2 g).The reactor was heated with heat transfer oil, and the heating temperature was set to 100°C.After the temperature is stabilized, the caprolactam solution and another raw material solution are respectively injected into the reactor through a metering pump, the flow rate of the caprolactam is 10ml/min, the flow rate of the acetic anhydride solution is 15ml/min, and the maintenance reaction residence time is 30 minutes.Samples were taken during the reaction, and the products in the reaction solution were analyzed by liquid chromatography-mass spectrometry.After the reaction was completed, the reaction solution was collected.The obtained reaction solution was distilled to remove solvent toluene, product acetic acid and unreacted acetic anhydride, and then distilled under reduced pressure to obtain 145.9 g of product. The yield of this reaction was 94%.
References:
CN114349702,2022,A Location in patent:Paragraph 0027-0030
7203-96-5
58 suppliers
$15.00/1g

1888-91-1
193 suppliers
$6.00/5g
2235-00-9
284 suppliers
$5.00/5g

1888-91-1
193 suppliers
$6.00/5g